Of the estimated 3 percent of the United States population with psoriasis, roughly 25 percent will develop psoriatic arthritis, with psoriasis of the nails or scalp disproportionately increasing the associated risk. The link between the two conditions has given researchers at NYU Langone Health a unique opportunity to study pre-clinical disease with a specific biomarker that could clarify how the inflammatory process progresses from skin to joints.
“We can’t turn the clock back once they progress to psoriatic arthritis … But we might be able to slow or stop progression to psoriatic arthritis through early treatment.”
Jose U. Scher, MD
“In patients with latent disease, we can’t turn the clock back once they progress to psoriatic arthritis, and our available medications all have limitations,” says Jose U. Scher, MD, director of the Psoriatic Arthritis Center. “But we might be able to slow or stop progression to psoriatic arthritis through early treatment.”
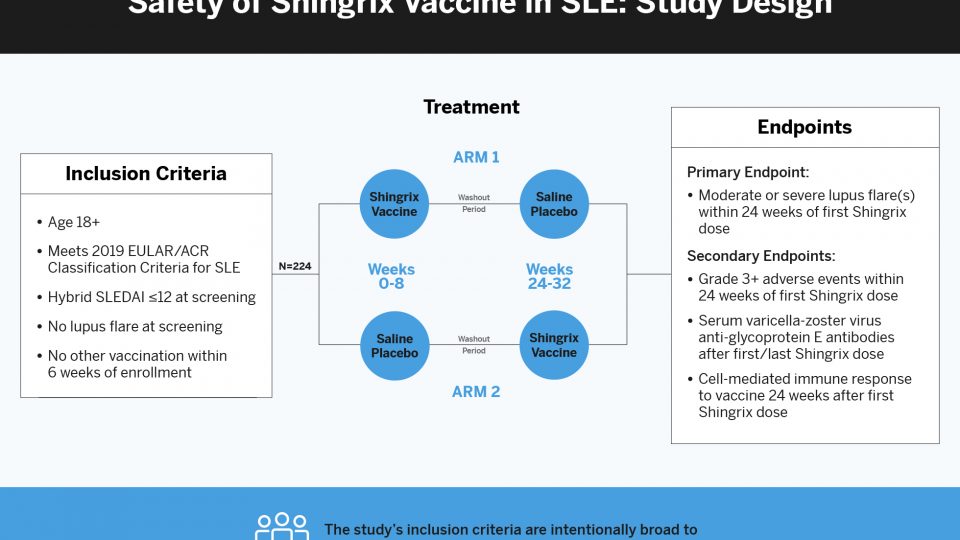
Dr. Scher and colleagues are leading a major multicenter study, Preventing Arthritis in a Multicenter Psoriasis At-Risk Population (PAMPA), that has been years in the making. The randomized trial across five North American medical centers has begun recruiting patients with psoriasis or other risk factors, such as a first-degree relative with psoriatic arthritis.
“We are focusing on the earliest treatment phase, before patients even develop joint pain,” says Rebecca Haberman, MD, the trial’s associate lead. “In addition to providing crucial insights into the progression of psoriatic disease, this study could change the paradigm for treatment.”
The study, conducted in collaboration with Janssen Pharmaceuticals, will test if a prolonged and unresolved skin inflammation, coupled with entheseal and synovial abnormalities detected via musculoskeletal ultrasound, will increase the risk for a transition to psoriatic arthritis.
Comparing Rates of Progression
In one arm, 100 at-risk patients will receive a subcutaneous injection of the drug guselkumab for 24 months. In a second arm, another 100 patients will be similarly randomized and begin the intervention or placebo after a 6-month delay. In a third arm of 150 patients who don’t wish to be treated, the researchers will use the non-biologic standard of care as a negative control.
“We’ll compare the rate of progression between those who are treated and those who are not treated,” Dr. Scher says.
Another primary endpoint will test whether the intervention improves subclinical inflammation within the first six months, as seen on ultrasound. In addition, the trial may reveal key biomarkers of progression, including whether the skin and gut microbiome, circulating immune cells, and molecules such as cytokines help predict progression from predominantly skin to joint inflammation.
Even if early intervention does not prevent outright progression from psoriasis to psoriatic arthritis, it might help attenuate the severity of disease.
“In addition to providing crucial insights into the progression of psoriatic disease, this study could change the paradigm for treatment.”
Rebecca Haberman, MD
“It may very well be that this is not the right drug to prevent progression,” Dr. Scher says. “But by looking at biomarkers, any implicated immune and environmental pathways might point toward cells or molecules that could be targeted in subsequent prevention efforts.”
Amping Up the Search for Better Therapeutics
The PAMPA prevention trial is being complemented by a larger National Institutes of Health (NIH)-funded program—the Accelerating Medicines Partnership (AMP).
Recently, AMP expanded its project on autoimmune disorders to include psoriatic disease as a major focal point. The public-private partnership is pairing rheumatologists and dermatologists from NYU Langone and other participating centers in the PAMPA study with the NIH and pharmaceutical companies to speed the development of better therapeutic options.
Part of the program’s focus will be on understanding the mechanics of disease progression, using data from the natural history of disease similar to the third arm of the PAMPA trial. In addition to tracking biomarkers such as potential changes in the microbiome and circulating immune cells, participants will undergo biopsies of the skin and joint synovium.
“The skin may have the code for who progresses, why, and how,” Dr. Scher says. “We want to find which cells that reside in the skin are being attracted—the term we use is homing—to the joints to produce and promote inflammation.”
Beyond identifying which patients progress, the larger research effort may clarify how the disease migrates and responds to therapy.
“Is there anything in the tissue that tells us whether some person will respond to medication X and not to medication Y, or vice versa?” Dr. Scher says. “What’s in that code may help us adopt a desperately needed precision medicine approach.”