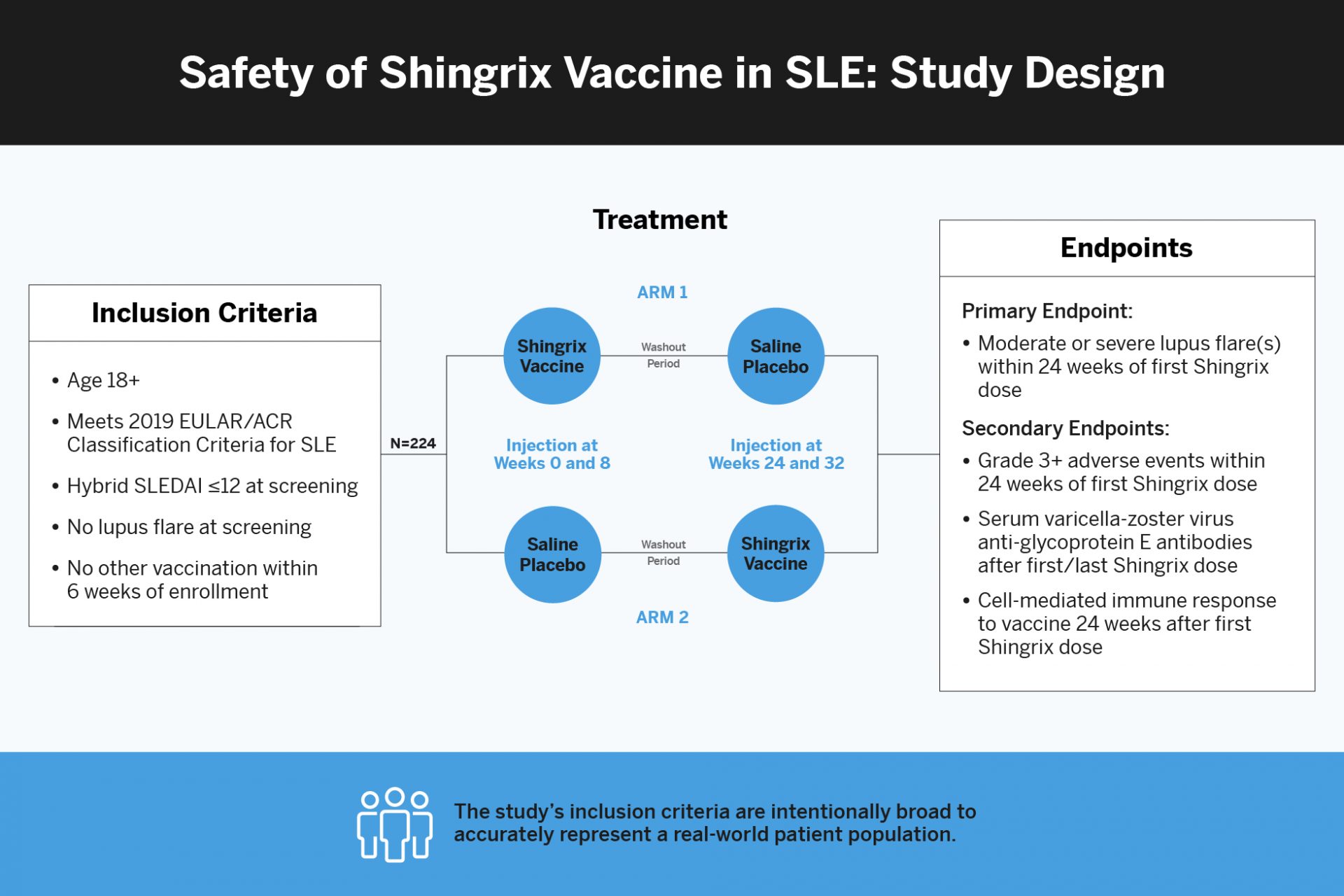
Herpes zoster, or shingles, is more common in patients with systemic lupus erythematosus (SLE) compared to the general population. An unresolved question has been whether the recombinant Herpes zoster subunit vaccine, Shingrix, is safe to use in patients living with lupus. A recently launched phase 4 clinical study, led by rheumatologist Amit Saxena, MD, an associate professor of medicine at NYU Langone Health, aims to evaluate the safety of Shingrix in patients with diverse baseline clinical activities, spanning different ages, and with varying levels of immunosuppressant exposures.
“Our goal is to establish the safety of the vaccine in a real-world patient population and gain a better understanding of the immune responses.”
Amit Saxena, MD
“Anecdotal reports suggest that the Shingrix vaccine poses no significant safety concerns for patients with stable lupus,” Dr. Saxena says. “Our goal is to establish the safety of the vaccine in a real-world patient population and gain a better understanding of the immune responses.”
The study, which is being offered at NYU Langone and the Oklahoma Medical Research Foundation, is open to adult patients with stable disease (i.e., no lupus flare(s) at the time of enrollment). Patients can be receiving many types of immunosuppressive or biologic agents for SLE treatment.
While accrual is at an early stage, Dr. Saxena and the research team plan to conduct an interim analysis in the second half of 2025.
They hypothesize that Shingrix administration will be non-inferior to placebo with respect to the risk of moderate or severe SLE flare(s) and that immunogenicity of the vaccine in lupus patients will be at least 50 percent of levels observed in healthy subjects from prior large clinical trials.
“We hope the findings will have therapeutic implications and help patients and their families feel more confident in their decision to vaccinate,” Dr. Saxena says.