Stress urinary incontinence (SUI) is extremely common, impacting millions of women. Some estimates suggest that up to 42 percent of women experience this condition in the United States1, increasing in prevalence as women age.
Despite the high prevalence of SUI, the substantial negative impact on quality of life, and the financial burden of the condition, data suggests that many women do not seek medical care. Embarrassment, lack of awareness of therapeutic options, and belief that urinary leakage is an inevitable part of aging are some of the contributing barriers.2
Here, we present the case of a 54-year-old woman who experienced success in managing her SUI with a new intravaginal insert, the Yōni.Fit device. The patient found the Yōni.Fit less intimidating than a surgical procedure, and it was less burdensome to identify the correct fit when compared to a traditional pessary.
Drawing from our center’s experience with prescribing modern intervaginal inserts for SUI, we believe raising awareness of their efficacy could encourage more women to seek help and discuss their condition.
Case Highlights:
- A 54-year-old patient experienced SUI for 15 years; Kegel exercises brought limited success, and shame deterred her from seeking care.
- The patient experienced immediate relief with the Yōni.Fit intervaginal insert, enabling her to resume an active lifestyle without fear of leakage.
- The Yōni.Fit is FDA-cleared, easy to self-fit at home, and reduces barriers for clinicians, requiring no inventory of multiple pessary sizes.
- NYU Langone led the pivotal clinical trial of the device, which demonstrated an 88.8 percent reduction in SUI events and high patient satisfaction.
Patient Case
The patient is a 54-year-old woman who has experienced bothersome urine leakage since the birth of her third child nearly 15 years ago. She reports leaking during most physical activities and when coughing, laughing, or sneezing. She has tried to stay physically active and regularly visits the gym. Recently, she started running again but found the activity no longer enjoyable due to severe leakage.
When discussing the impact of SUI, she admits to avoiding exercise classes after an embarrassing incident that left her pants soaking wet. Having entered menopause, she confesses feeling depressed about aging. Over the years, she has performed Kegel exercises, but she no longer continues them, as they frustrated her since she could not become pad-free.
She admits to not mentioning the effects of the leakage due to shame and acknowledges that healthcare providers did not inquire about her condition.
She recalls reading about surgeries during her online searches years ago, but she was fearful of potential complications and recovery time postsurgery.
SUI Treatments
Although midurethral sling surgery is the gold standard for SUI, the American Urological Association (AUA)/Society of Urodynamic, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) guideline on the surgical treatment of SUI acknowledges that not every patient is an appropriate candidate for treatment with surgical intervention.3 The guideline states, “Pelvic floor muscle training and incontinence pessaries are appropriate for patients interested in pursuing therapy that is less invasive than surgical intervention.”
Pessaries can be very effective for some women with SUI. One drawback of traditional anti-incontinence pessaries is the time and effort needed to find an appropriate fit in the office. This is particularly burdensome when it is not a regular part of the provider’s practice. The office requires specific equipment, exam room time, and an inventory of multiple shapes and sizes of anti-incontinence pessaries. Anti-incontinence pessaries also require a particular orientation (with the knob compressing the urethra) that can make self-care (removal and replacement) more difficult.
The evolution of the more traditional anti-incontinence pessary to a more patient-friendly intravaginal insert allows for a device that can be inserted with less clinician involvement: it’s also easier for women to use. Unfortunately, efficacy and comfort have been less than desired. Level-one evidence is lacking for many newer intravaginal inserts, and thus real-life efficacy has fallen below expectations.
Yōni.Fit: The Trial
Experts from our Division of Female Pelvic Medicine and Reconstructive Surgery helped develop the protocol and design the pivotal clinical trial for the novel intravaginal insert the Yōni.Fit. In addition, NYU Langone served as the lead study study site for the randomized, sham-controlled, single-blind, multicenter study.
Between 2020 and 2022, the trial screened 79 women who were able to self-report SUI and SUI-predominant mixed incontinence, and 58 were subsequently randomized. In a decision that was unique among tests for vaginal inserts, this trial design used a sham device, a silicone ring resembling those used for continuous vaginal estrogen administration. All cohort sites were demographically similar in the treatment and sham arms. The average age across all sites was 46.5 ± 10.6 years, with a mean BMI of 27.8. Both pre- and postmenopause women were included.
All subjects were able to self-fit the devices. The primary outcome—a rigorous 12-hour pad weight assessment—demonstrated a mean decrease of 56.2 percent and a reduction of the SUI events of 88.8 percent. Patients were also asked about global outcome measures (Patient Global Impression of Change, PGIC) and 82 percent were noted to be “much improved” or “better.” Women using the Yōni.Fit device had double the pad dry days compared to the sham arm. The adverse events were mostly considered mild or moderate.
The study resulted in FDA clearance for the device in May 2024.
Yōni.Fit: Prescribing the Device
The Yōni.Fit is now available to simplify the process of intravaginal inserts and anti-incontinence pessaries. The clinician simply performs a vaginal exam confirming SUI.
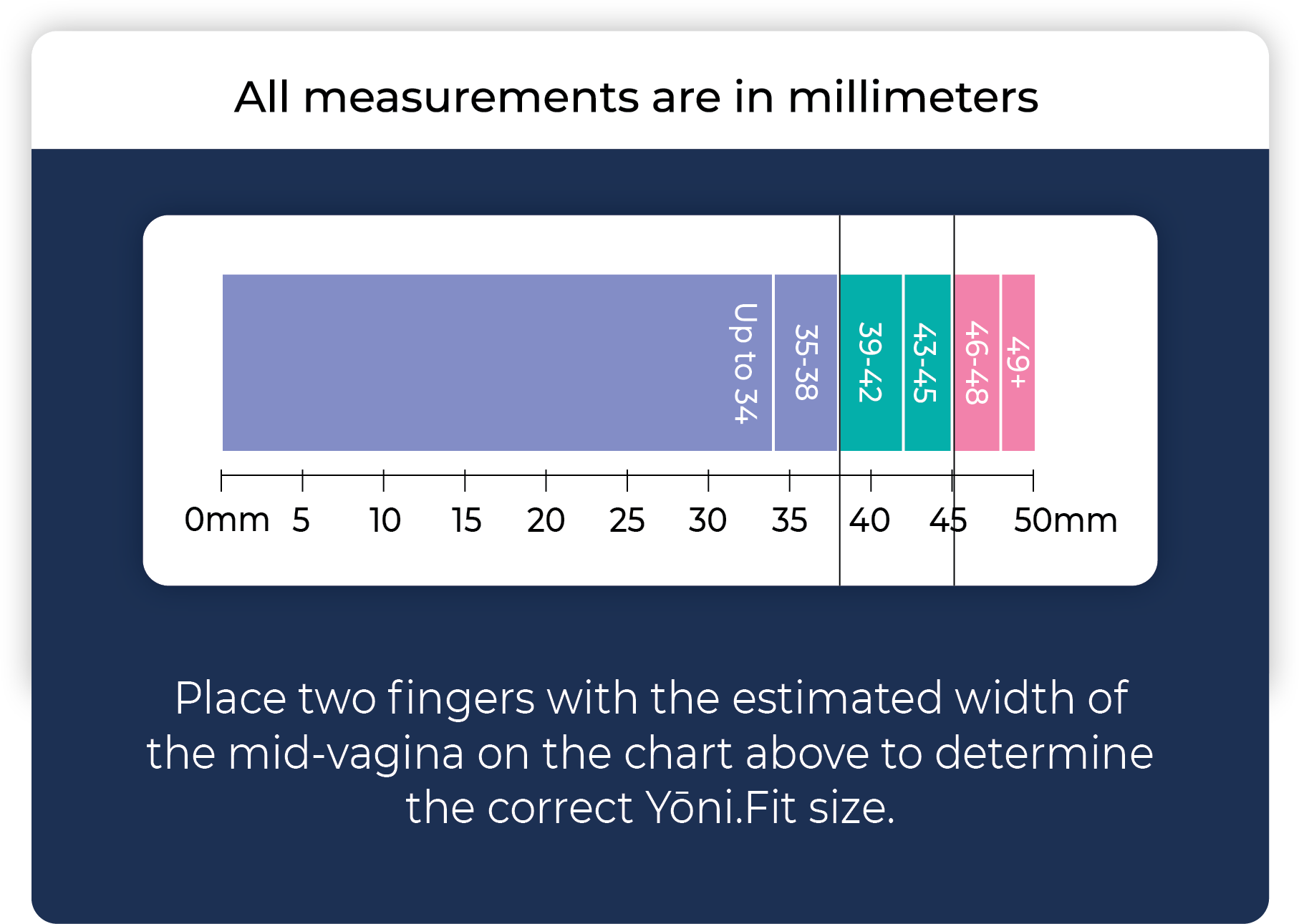
By placing two fingers in the vaginal canal, the estimated width of the vagina at the midpoint between the urethra and the cervix or vaginal cuff is determined. The measurement is compared to a measurement reference chart, which offers a clear range to select the appropriate fit kit size (Figure 1).
Check with your practice billing experts, but it is suggested that CPT code 57160 be used for this fitting of pessary.
A prescription for one of three size kits is sent to a pharmacy that will distribute the kit, which includes two Yōni.Fit sizes, a carrying case, and a cleaning brush. In the comfort of her home, a patient can try the different sizes to find the best fit. If there is any discomfort, another fit kit can be dispensed.
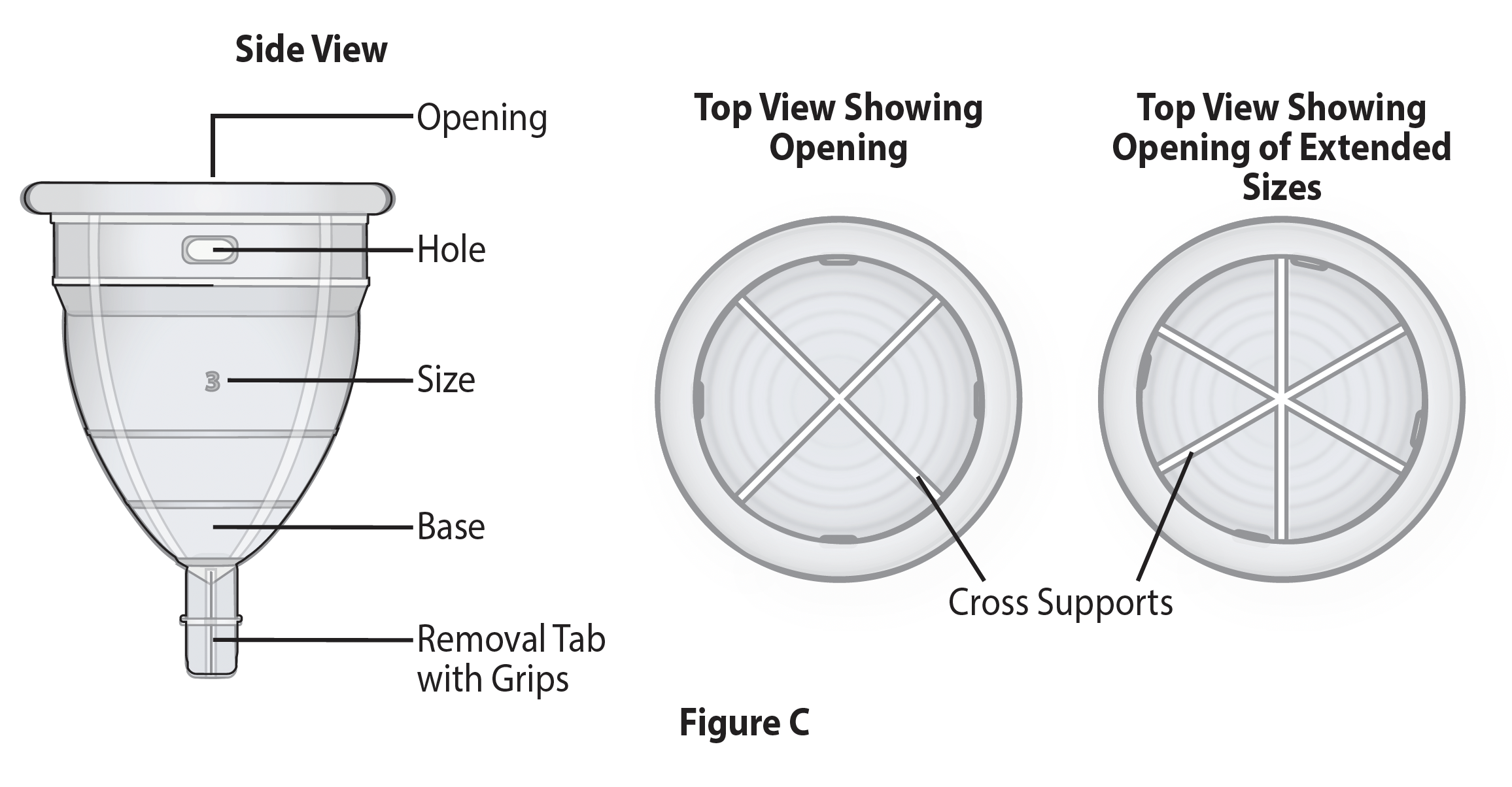
The device is symmetrical, avoiding the need to rotate to insert to a specific orientation for it to be effective. The removal tab was designed with ease of removal in mind. The device is made of surgical-grade silicone that is compressible, aiding in the comfort of placement and removal. However, the design (i.e., support struts and the location of compression at the bladder neck) contributes to the profound efficacy of this device (Figure 2).
In most cases, A4564 allows the pharmacy to bill the patient for the device. With no coverage, a single reusable insert costs about $60.
Conclusion
SUI impacts millions of women in the United States, but many women do not seek care due to embarrassment or misconceptions. Intravaginal inserts and pessaries offer a less-invasive option for managing SUI compared with surgery. Their favorable side-effect profile makes them appealing to many women.
In some instances, patients may seek care from providers who do not personally perform anti-incontinence surgeries but instead refer patients to experts in incontinence. For both these providers and specialists in incontinence, intravaginal devices can provide a temporary, nonsurgical option, helping patients understand that there is no longer a reason to remain silent about their condition and its effects. Therefore, an intravaginal insert like the Yōni.Fit, which requires little in-office specialized equipment and no need to carry an inventory of different shapes or sizes, may serve to reduce barriers and increase the number of women who come forward to discuss their condition.
Our 54-year-old patient considered the Yōni.Fit less intimidating than a surgical procedure, with minimal hassle for the provider and little cost to the practice. She now uses the device most mornings, when she is most active, and reports that she now feels in control of her life. She no longer experiences leakage or has the fear of leaking. The device provides immediate relief, while not ruling out other treatments, giving her confidence and demonstrating that managing SUI goes beyond simply using pads to hide the leakage.
Disclosures
Dr. Brucker contributed to the design of the Yōni.Fit clinical trial (NCT03978741) as well as the data analysis of the trial outcomes as a medical monitor.
References
- Burton CS, et al. Curr Urol Rep. 2022;23(9):185–194. DOI.
- LaPier Z, et al. Urogynecology (Phila). 2024;30(3):352–362. DOI.
- Kobashi KC, et al. J Urol. 2017;198(4):875–883. DOI.
- Escobar C, et al. Randomized Controlled Trial Evaluating Efficacy and Safety of a Novel Stress Incontinence Device. Abstracts 24 AUGS 2023. Urogynecology. 2023 Oct 29(10S):S56–S57.