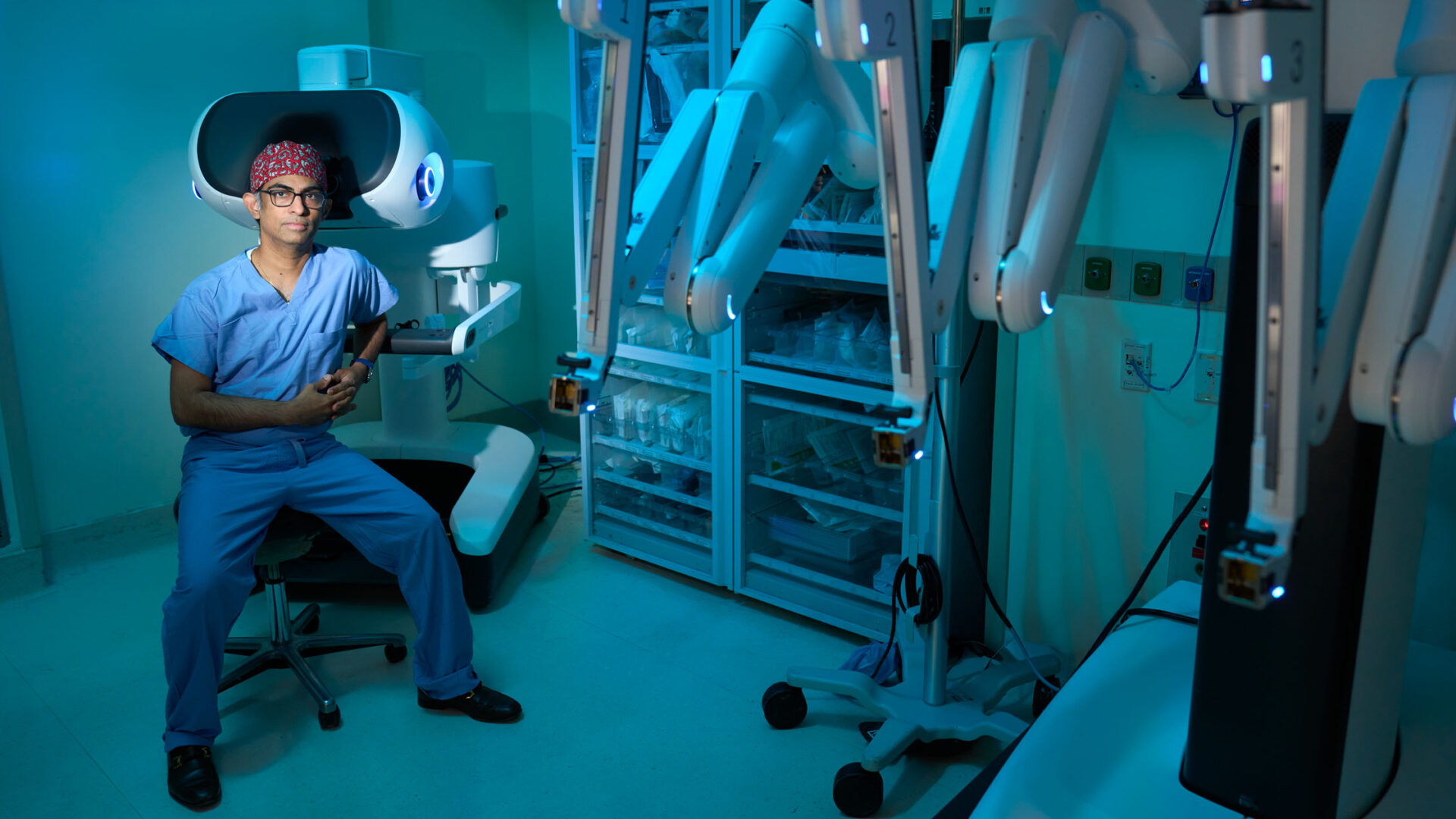
Umamaheswar Duvvuri, MD, PhD—who has performed roughly 600 robotic head and neck surgeries—is the first surgeon in New York State to use the new da Vinci 5 to treat patients with head and neck lesions. Here, the chair of the Department of Otolaryngology—Head and Neck Surgery at NYU Langone Health describes the potential benefit of the latest da Vinci robotic system, explaining how expanding its use in head and neck surgery could contribute to FDA approval for that indication.
Physician Focus: NYU Langone Health is one of the select few centers in the nation using the da Vinci 5 for head and neck surgeries. How does this latest version of the system differ or improve upon other robotic systems?
Dr. Duvvuri: The main improvement is that this newest version incorporates technology for force feedback, allowing the surgeon to feel the amount of force applied when manipulating tissue. We hope that this will help to enhance safety over time, not only by offering tactile feedback to understand how much force is needed, but also by helping the surgeon to better discriminate between soft tissue and hard tumors during surgery.
“We hope that [force feedback] will help to enhance safety over time by helping the surgeon to better discriminate between soft tissue and hard tumors.”
Umamaheswar Duvvuri, MD, PhD
Physician Focus: How do you evaluate which patients are good candidates for the da Vinci 5 system?
Dr. Duvvuri: It’s really about which patients are good candidates for robotic surgery, regardless of the specific system we use. We recommend robotic surgery for a range of malignant or benign head and neck tumors, particularly those tumors located in a hard-to-reach area, such as the oropharynx. The robotic approach often enables us to de-escalate adjuvant treatments for malignancies, while offering improved safety and cosmetic outcomes. Once a patient is determined to be eligible for robotic surgery, we can opt for the da Vinci 5 or for the traditional robots.
Physician Focus: The da Vinci 5 is FDA-approved for gynecologic, urologic, and general laparoscopic and thoracoscopic procedures, but hasn’t yet been explicitly approved for ENT surgeries. What is needed to gain FDA approval for ENT applications?
Dr. Duvvuri: The entire story of robotic surgery has been one of evolution; in fact, the da Vinci system was originally built as a remote system to treat wounded soldiers on the battlefield without having to deploy a surgeon. It was then adapted for minimally invasive surgery on the prostate and abdomen, and in the uterus. The new indications were all found through innovation.
“Our goal is to collect initial outcomes data from 10 or more patients, then lead a trial that randomizes patients to the da Vinci 5 or da Vinci Xi to compare their outcomes.”
That is what we’re doing with the da Vinci 5—we’re demonstrating its use in head and neck surgery so we can push the field forward. If we can show safety and efficacy for more patients with this indication, we hope to expand the system’s adoption and, ultimately, obtain specific FDA approval for the da Vinci system in the resection of head and neck tumors.
Physician Focus: How do the data look so far—how many patients have you treated with the da Vinci 5, and how are they doing?
Dr. Duvvuri: We’ve treated about six patients so far, with more cases planned in the near future. All treated patients are doing well, with no untoward complications. I’ve performed hundreds of robotic surgeries in my career, so given the specific updates to the da Vinci 5 over earlier versions, I wouldn’t expect major safety concerns.
That said, we will follow these patients and examine safety and outcomes over the long term. Our goal is to collect initial outcomes data from 10 or more patients, then lead a trial that randomizes patients to the da Vinci 5 or da Vinci Xi to compare their outcomes. We will publish and share these data with the field so we can collectively look at whether the da Vinci 5, and any future versions, meaningfully improves outcomes for patients compared with longer-established robotic technology.