Managing the recurrence of urethral stricture after urethroplasty can be challenging. The Optilume drug-coated balloon, which delivers paclitaxel to the urethral wall to, in theory, prevent recurrence, is a promising novel treatment for urethral stricture. Although randomized studies have demonstrated the safety and effectiveness of Optilume urethral dilation for treating recurrent strictures after endoscopic management, there have been no published studies on its efficacy following open urethroplasty.
The following Case of the Month demonstrates the application and potential utility of Optilume urethral dilation for endoscopic management of recurrent urethral stricture following urethroplasty.
Since the relative rarity of stricture recurrence after urethroplasty makes patient recruitment in clinical trials a challenge, peer-to-peer discussion on the application of Optilume dilation after urethroplasty may help advance treatment and improve patient care.
Case Highlights:
- The Optilume drug-coated balloon is approved by the FDA for treatment of anterior urethral strictures that are less than or equal to 3 cm long.
- The device has demonstrated success in treating recurrent strictures after endoscopic management. To date, there are no published data on its effectiveness for recurrence after urethroplasty.
- In the case presented, a 45-year-old male experienced lasting relief after electing Optilume dilation for a 1-cm proximal apical recurrence following urethroplasty for a 5-cm stricture.
- Further demonstrations of the success of Optilume in managing similar cases may support its use following urethroplasty.
Patient Case
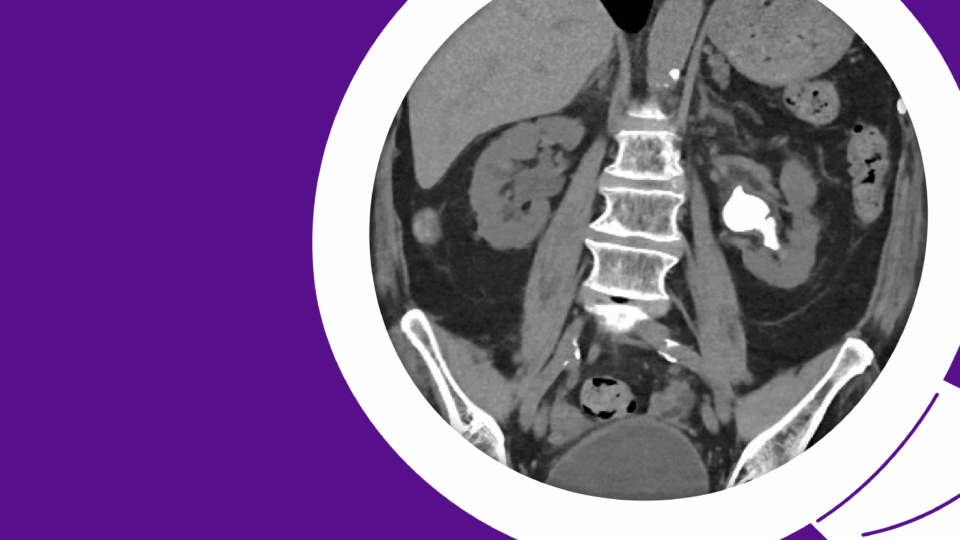
The patient first presented to his primary care physician with severe lower-urinary-tract symptoms (LUTS), including straining, weak stream, and hematuria. Though the patient initially attributed his condition to diet, his AUA Symptom Score of 33 represented severe LUTS. His post-void residual (PVR) was 450 ml, and uroflow measured as follows: max flow rate 5.6 ml/second, average flow rate 2.2 ml/second, 138 cc of voided volume. Upon referral to urology, retrograde urethrogram demonstrated a long-segment (5 cm) bulbar urethral stricture (Figure 1).
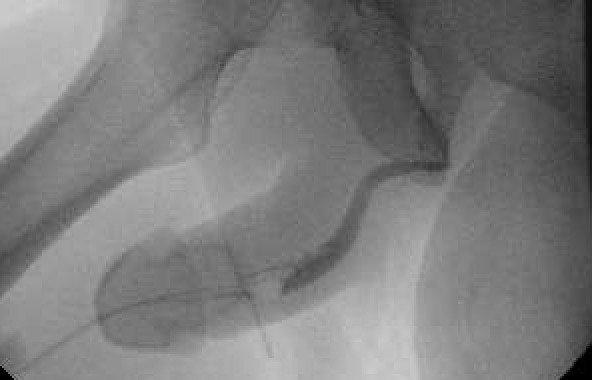
At this point, the patient presented to NYU Langone Health, where we discussed management options, including uncoated-balloon catheter urethral dilation or urethroplasty: Optilume was not yet FDA-approved for treatment of urethral strictures. The patient understood the high probability of recurrence following dilation, based on the length of his stricture, and elected dorsal buccal graft onlay urethroplasty.
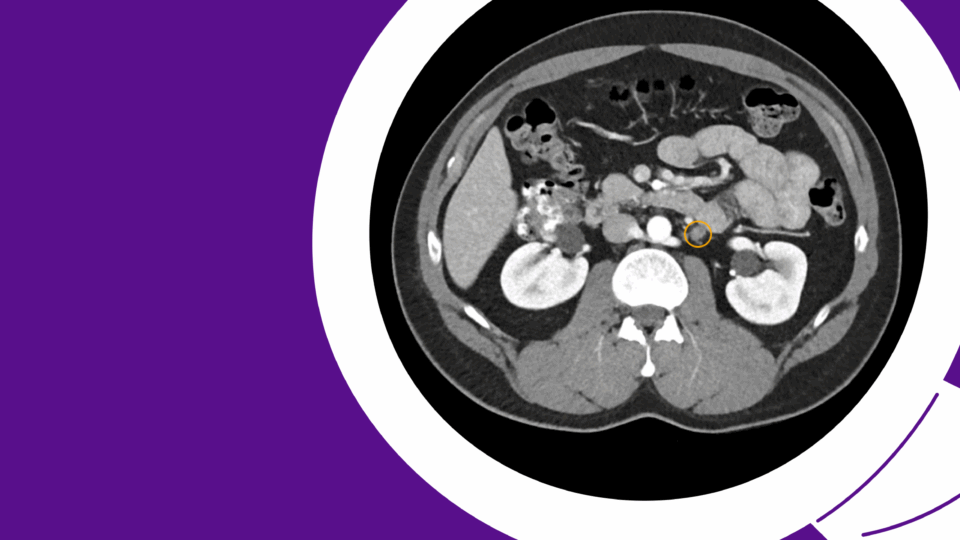
One year later, the patient presented with worsening LUTS, including progressive diminution of his urinary stream, and had a PVR of 125 ml. Uroflow measured as follows: max flow rate 5 ml/second, average flow rate 3 ml/second, 150 cc voided volume. Cystoscopy confirmed a recurrent 1-cm, 5-Fr urethral stricture at the proximal apex. At this time, we counseled the patient on options, including repeat urethroplasty and urethral dilation with or without Optilume, and he chose Optilume dilation.
It has now been one year since the procedure, and the patient continues to have a durable result.
Optilume Dilation: The Procedure
The procedure was performed in the operating room under general anesthesia per initial guidelines. However, Optilume can now be performed in the ambulatory clinic. A flexible cystoscope was advanced into the urethra to the 5-Fr bulbar urethral stricture, confirmed to be 1 cm long. A 0.035 sensor wire was advanced into the bladder, and a 24-Fr uncoated dilating balloon catheter was advanced over the wire.
Under direct visualization, the uncoated dilating balloon catheter was advanced to the urethral stricture and inflated to 20 mmHG. The balloon catheter was removed and a 24-Fr Optilume dilation balloon was advanced under direct vision to the urethral stricture and inflated for a five-minute dwell time. The cystoscope and the Optilume drug-coated balloon catheter were removed. A 16-Fr council Foley was advanced over the wire and into the bladder and connected to gravity drainage.
Following the uncomplicated procedure, the patient was discharged and returned 72 hours later for Foley removal. Optilume care instructions include abstinence or using a condom for 30 days after treatment to avoid exposure of a partner to paclitaxel, and abstinence or contraception with sexual partners of childbearing age for at least six months after treatment to avoid potential risks to a fetus.
Optilume Dilation: Evidence and Outcomes
Evidence supporting Optilume utilization is from ROBUST III, a prospective, randomized, multicenter, single-blind trial that compared Optilume to traditional methods of endoscopic management of anterior urethral stricture.1,2 Eligible participants were adult males with strictures less than or equal to 3 cm in length who had had at least two prior endoscopic treatments.
Participants in the intervention arm were pretreated with an uncoated balloon or direct vision internal urethrotomy to greater than or equal to 20 Fr. An Optilume drug-coated balloon was administered, with balloon sizes selected based on lumen diameter and stricture length. Inflation with the Optilume drug-coated balloon for five minutes or more allowed delivery of paclitaxel, and then a Foley catheter was placed for two to five days.
At two years, patients who underwent Optilume dilation demonstrated sustained improvements in LUTS severity, captured using the International Prostate Symptom Score, and voiding parameters. When evaluating additional interventions, patients in the Optilume arm at two years had fewer repeat interventions than those in the control arm at one year (77.8 percent versus 23.6 percent).
Discussion
In summary, Optilume is established as a safe and effective endoscopic treatment for those who wish to avoid or delay formal urethroplasty. In this Case of the Month, we also illustrate how Optilume urethral dilation is a safe and effective option to consider for patients who present with recurrent strictures after urethroplasty.
Endoscopic management of recurrent urethral strictures following bulbar onlay urethroplasty is feasible.3,4 Almost half of these recurrences occur at anastomotic sites, and success rates of direct vision internal urethrotomy for short recurrent strictures is 60.5 percent. Recurrence after a trial of endoscopic treatment, however, should be managed with repeat open reconstruction.5
It is important to note that there is no consensus on the ideal definition of success after urethroplasty. Some have considered freedom from re-intervention for urethral stricture as success, though others point out that certain patients with recurrences may elect no further intervention.6
Another definition, established by the Trauma and Urologic Reconstructive Network of Surgeons (TURNS), is the ability to pass a 16-to-17-Fr flexible cystoscope beyond the area of reconstruction.7 This definition is challenging, because routine endoscopy on all patients who undergo urethroplasty is not feasible due to costs and lack of evidence of its impact on further patient management.8
Another definition is based on the postoperative maximal flow rate (Qmax), but this measure is multifactorial, and a high Qmax does not rule out recurrence.9 A final definition is freedom from LUTS or an improvement compared to baseline.
New tools, including the Urethral Stricture Surgery (USS) patient-reported outcome measure, and a reduced version called the Urethral Stricture Symptom and Impact Measure (USSIM), have been used to evaluate symptoms.10,11 These new tools, though important and necessary, also bring new challenges for utilization.
Ultimately, the definition of success after urethroplasty should come from the patient. As a provider, I discuss with each patient what successful treatment of their stricture would look like and identify the best approach to achieve those goals. In the case presented here, the patient defined success as no longer straining to urinate, having a steady stream, and being able to empty his bladder. These results were achieved with Optilume dilation.
References
1. Elliott SP et al. (2022). One-Year Results for the ROBUST III Randomized Controlled Trial Evaluating the Optilume Drug-Coated Balloon for Anterior Urethral Stricture. J Urol; 207(4): 866–875. DOI.
2. VanDyke ME et al. (2024). Optilume Drug-Coated Balloon for Anterior Urethral Stricture: 2-Year Results of the ROBUST III Trial. BJUI Compass; 5(3): 366–373. DOI.
3. Barbagli G et al. (2006). Anastomotic Fibrous Ring as Cause of Stricture Recurrence after Bulbar Onlay Graft Urethroplasty. J Urol; 176(2): 614–619; discussion 9. DOI.
4. Rosenbaum CM et al. (2015). Internal Urethrotomy in Patients with Recurrent Urethral Stricture after Buccal Mucosa Graft Urethroplasty. World J Urol; 33(9): 1337–1344. DOI.
5. Zaid UB et al. (2016). Management of the Recurrent Male Urethral Stricture. Curr Urol Rep; 17(4): 33. DOI.
6. Meeks JJ et al. (2009). Stricture Recurrence after Urethroplasty: A Systematic Review. J Urol; 182(4): 1266–1270. DOI.
7. Erickson BA et al. (2014). Multi-Institutional 1-Year Bulbar Urethroplasty Outcomes Using a Standardized Prospective Cystoscopic Follow-Up Protocol. Urology; 84(1): 213–216. DOI.
8. Baradaran N et al. (2019). Clinical Significance of Cystoscopic Urethral Stricture Recurrence after Anterior Urethroplasty: A Multi-Institution Analysis from Trauma and Urologic Reconstructive Network of Surgeons (TURNS). World J Urol; 37(12): 2763–2768. DOI.
9. Tam CA et al. (2016). Critical Analysis of the Use of Uroflowmetry for Urethral Stricture Disease Surveillance. Urology; 91: 197–202. DOI.
10. Breyer BN et al. (2017). Comprehensive Qualitative Assessment of Urethral Stricture Disease: Toward the Development of a Patient-Centered Outcome Measure. J Urol; 198(5): 1113–1118. DOI.
11. Jackson MJ et al. (2011). Defining a Patient-Reported Outcome Measure for Urethral Stricture Surgery. Eur Urol; 60(1): 60–68. DOI.