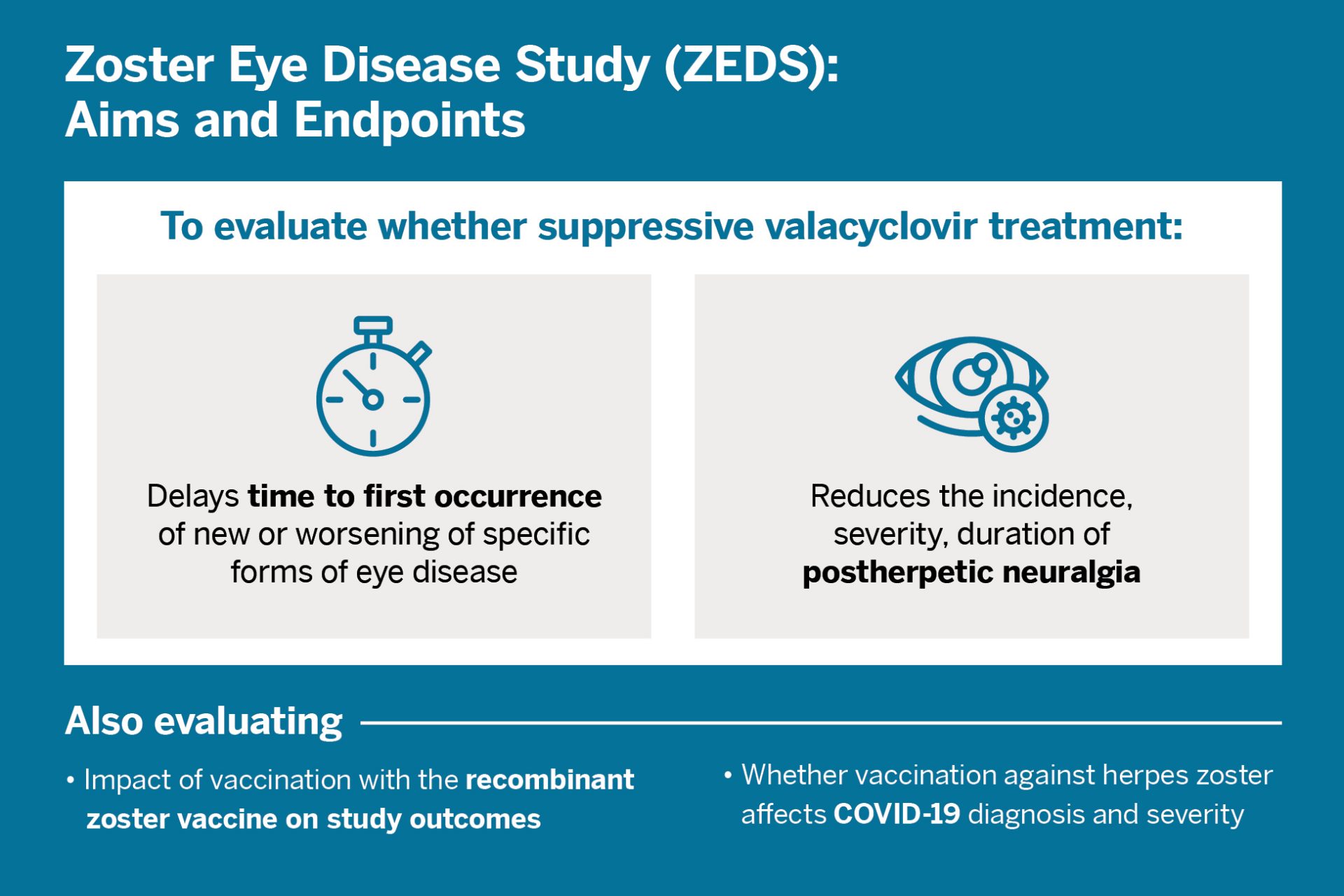
Current standard treatment for herpes zoster ophthalmicus (HZO), or shingles of the eye, involves short-term use of a high oral dose of an antiviral valacyclovir, acyclovir, or famciclovir. An unresolved question has been whether longer-term valacyclovir treatment can improve outcomes in HZO and should become standard of care.
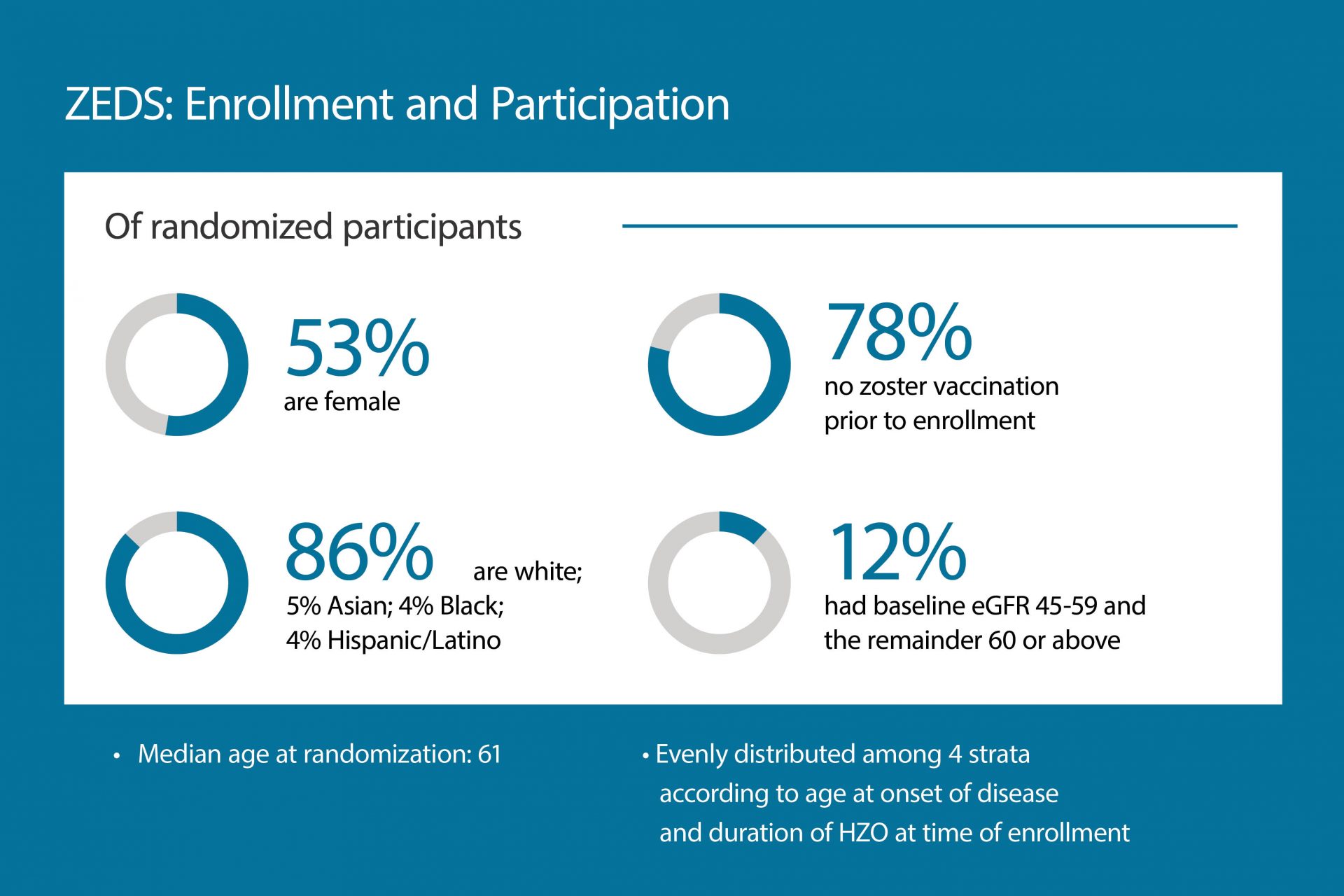
In 2016, Elisabeth J. Cohen, MD, a professor of ophthalmology, and her team were awarded a $15 million grant from the National Eye Institute of the National Institutes of Health to explore long-term use of valacyclovir. The resulting 75-center Zoster Eye Disease Study (ZEDS) is currently ongoing.
“Our goal is to reduce recurrant and chronic eye disease and chronic pain due to HZO.”
Elisabeth J. Cohen, MD
“We already know that long-term suppressive treatment has greatly improved outcomes for herpes simplex eye disease, which shares key similarities to herpes zoster eye disease,” says Dr. Cohen. “Our goal is to reduce recurrent and chronic eye disease and chronic pain due to HZO.”
A Vision for Treatment Born from Experience
A cornea specialist, Dr. Cohen suffered from herpes zoster eye disease in 2008. She lost depth perception and had to give up her practice. Always interested in eye infections, she refocused her research toward finding a better treatment for HZO.
Dr. Cohen has been a tireless advocate in her efforts to promote the herpes zoster vaccine. “We now have the recombinant zoster vaccine that is over 90 percent effective,” she says. “It is my ambition that we get as many people as possible age 50 and older and immunocompromised adults protected from this common, debilitating disease that is vision- and life-threatening.”
Sustaining ZEDS Research through COVID-19
While the pandemic derailed many clinical trials requiring participant visits, ZEDS notably maintained its momentum. Dr. Cohen and the ZEDS research team outlined their successful strategy to retain participants and quickly resume enrollment in Contemporary Clinical Trials Communications. The team also outlined the study design and enrollment progress in a recent issue of Cornea.
“Through the unwavering commitment of researchers here at NYU Langone and at the participating clinical centers, we have been able to sustain our enrollment,” Dr. Cohen notes. “We hope our work can provide insights into the planning of future trials in addition to improving outcomes in HZO.”
Dr. Cohen and the research team encourage all doctors to refer patients with HZO to a study site near them for evaluation, or to contact the NYU Grossman School of Medicine by phone at 844-NYU-ZEDS or by email at ZEDS.CTA@nyulangone.org.