New research published in the Annals of Rheumatic Diseases sheds light on the role that a specific gut bacterium may play in triggering flares and serious symptoms in people with severe systemic lupus erythematosus (SLE).
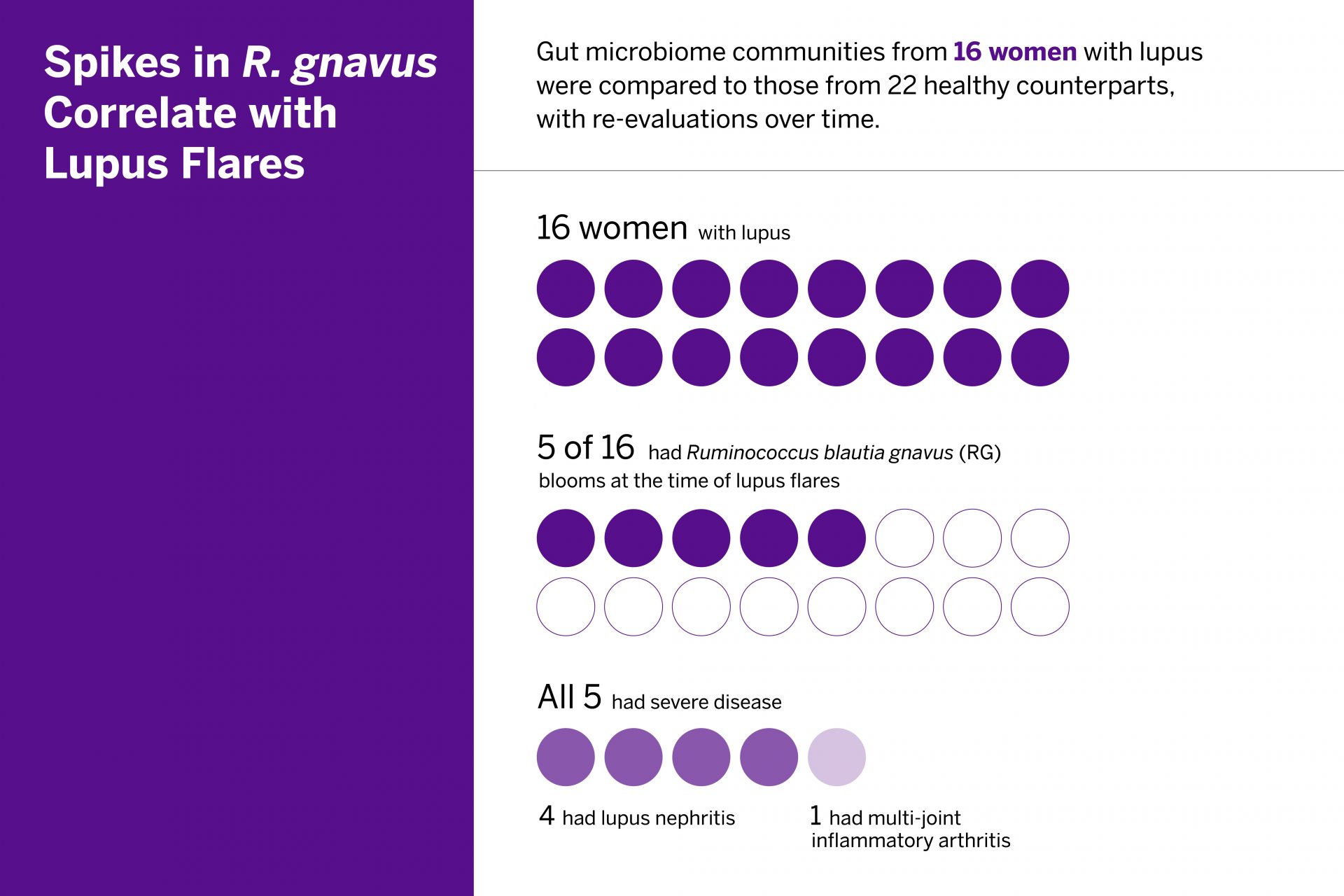
In the study, researchers observed that significant blooms of Ruminococcus (blautia) gnavus (RG) are common during high-disease activity but never seen in healthy counterparts. They also identified an immunologic response to the bacterium that may contribute to the inflammation associated with disease flare-ups.
Prior research by lead investigator and rheumatologist-immunologist Gregg Silverman, MD, has shown that RG blooms themselves weaken the gut wall barrier, prompting the release from the gut of bacterial factors that stoke an overactive immune response and inflammation.
“These findings are the strongest to date showing the silent growth of the pathobiont R. gnavus is a disease driver of active flares in patients with lupus nephritis.”
Gregg Silverman, MD
“These newest findings are the strongest to date showing the silent growth of the pathobiont R. gnavus is a disease driver of active flares in patients with lupus nephritis,” says Dr. Silverman.
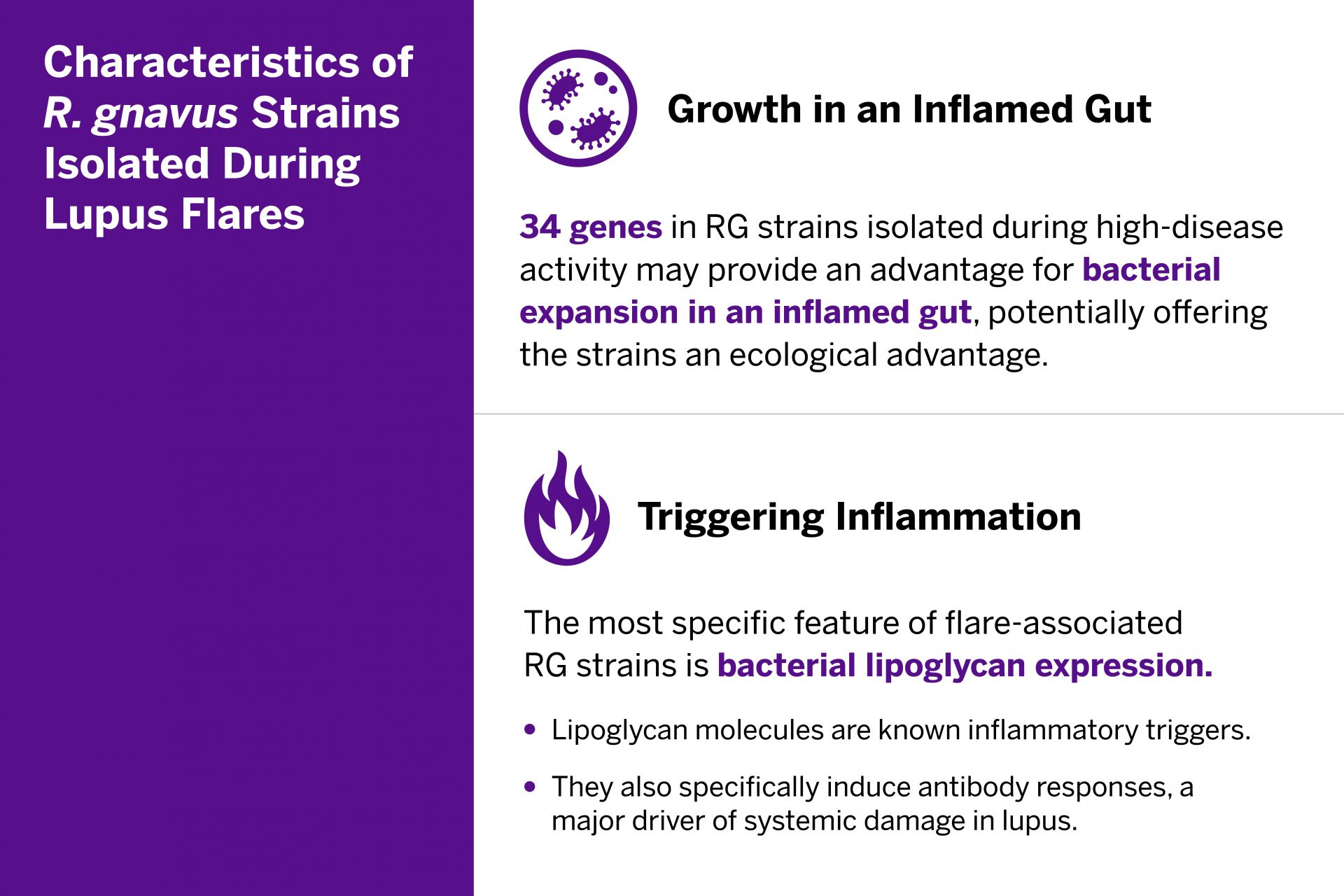
The RG gut bacterial blooms appear to trigger a cascade of immunologic activity associated with inflammation. From the RG strains isolated during flares, researchers identified 34 genes with established links to the bacterium’s growth in patients with inflammation. The team also investigated the patients’ immune response to the bacteria and isolated specific bacterial lipoglycans known to trigger inflammation. The lipoglycans were common in RG strains in patients with lupus, but not in the study’s healthy control group.
The presence of RG blooms concurrent with lupus flare-ups, along with the immune responses to the RG bacteria, suggests a biological pathway for lupus that could be targeted with future treatments.
“As we continue to uncover how lipoglycans from bacteria trigger an immune response—and, in turn, inflammation—the hope is that we might turn to less toxic antibacterial therapies that target bacterial imbalance rather than dampen the immune system that protects us from infections like COVID,” Dr. Silverman says.