Advances in genetic engineering and immunosuppression have raised hopes that genetically modified pigs might help solve the chronic shortage of transplant organs for human patients.
Although xenotransplantation of hearts from such animals has shown encouraging results in nonhuman primates, it has been attempted only twice in a living person—in January 2022 and September 2023, when patients at the University of Maryland received pig hearts under the FDA’s “compassionate use” provision. The first patient survived for 60 days before the organ failed; the second patient was still being monitored at the time of publication.
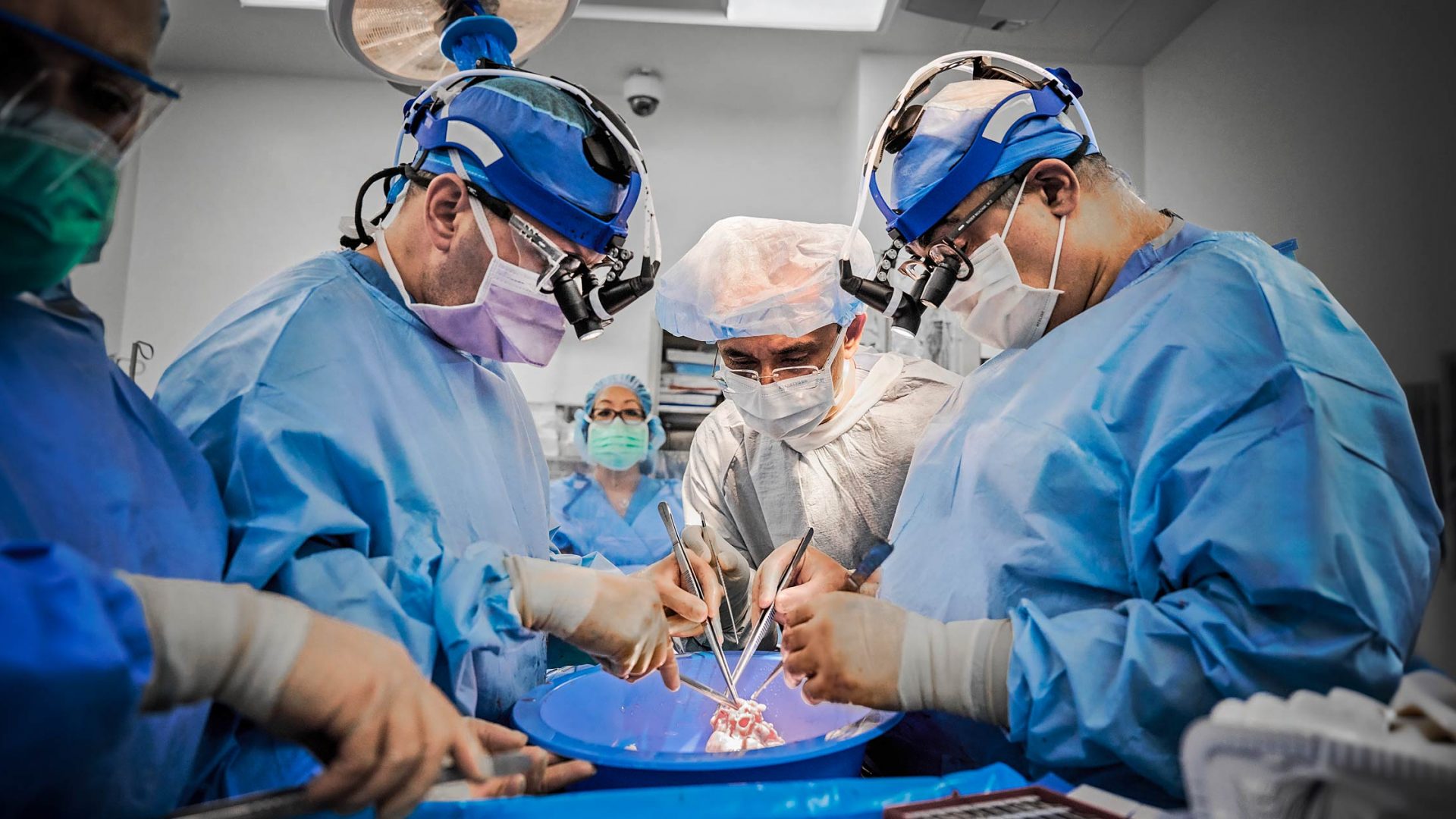
NYU Langone Health researchers are pioneering in this emerging field of innovation by taking a different approach: being the first to transplant gene-edited pig hearts into recently deceased humans. Led by Nader Moazami, MD, chief of the Division of Heart and Lung Transplant and Mechanical Circulatory Support, they recently reported findings in a study published in Nature Medicine. The transplant recipients were a man and a woman, both of whom had consented to full-body donation. Heart function was monitored for three days.
Here, Dr. Moazami discusses the study’s methodology and findings, and its implications for the future of cardiac xenotransplantation.
Pioneering the Decedent Model
Physician Focus: Dr. Moazami, why did you decide to use decedent recipients in this study?
Dr. Moazami: The big question in xenotransplantation is whether the lessons we’ve learned from pig-to-primate transplants can be applied to humans. Our goal was to investigate that question without jeopardizing a recipient’s life. My colleague Dr. Robert Montgomery, director of the NYU Langone Transplant Institute, has pioneered the decedent model in pig-to-human kidney xenotransplantation, with great success. We thought it would be useful to take a similar approach with the heart.
“We’ve never been as close to making xenotransplantation a reality as we are now.”
Nader Moazami, MD
Based on discussions with Dr. Montgomery, we set out to determine three things: Is the pig heart robust enough to maintain blood flow in the human? What happens to this heart immunologically? And are any viruses transmitted from the pig to the human? With a deceased recipient, we could investigate those issues in much more detail than with a living patient—for example, by doing frequent biopsies and multiple daily blood draws.
Navigating Complex Logistics
Physician Focus: How did you carry out the experiments?
Dr. Moazami: Several months of planning were required. We needed to work out what kind of consent we were going to use and how we would approach the families. We needed to have an OR available, which would then become an ICU, for a three-day period. We needed to train our surgical team in the unique challenges of xenotransplantation. We had to rehearse the procurement protocol, which included transporting the donor heart from a special pig-breeding facility in Virginia.
We used animals grown under sterile conditions with 10 genetic modifications—4 genes knocked out to eliminate immunogenic sugars and keep the heart from growing too large, and 6 human genes inserted to improve immunocompatibility. We also used an extremely sensitive screen for porcine viruses to reduce the risk that latent viruses might be present in the donor heart.
Once appropriate recipients became available—decedents with organs that were ineligible for donation but whose physiology could withstand the rigors of transplant surgery—we performed the implantations just as we would with a human heart. Afterward, both recipients were monitored 24 hours a day by a team of intensivist physicians and nurses, as well as by the research team.
Encouraging—and Instructive—Results
Physician Focus: Tell us about the results. Were there any surprises?
Dr. Moazami: Overall, the results were very positive. There was no sign of rejection or inflammation. The hearts kept pumping without the need for mechanical support. And we detected no porcine viruses in the hearts or in the recipients.
However, there were differences in outcome between the two xenotransplants. In the first case, there was a significant size mismatch between the donor heart and the recipient, who had previously had cardiomyopathy. The recipient needed high doses of vasopressors and inotropes, and, by the third day, we saw a deterioration of cardiac output. In the second case, where the size match was good, none of those problems occurred.
“For pig-to-human cardiac transplants to become clinically feasible, we’ll need a better way to match a potential donor pig with a prospective recipient’s anatomy.”
One takeaway is that for pig-to-human cardiac transplants to become clinically feasible, we’ll need a better way to match a potential donor pig with a prospective recipient’s anatomy. Our team has launched another study in which we’re monitoring the growth of the heart at different stages of the animal’s lifetime to get a better understanding of the relationship between heart size and body weight.
Physician Focus: What else does this study tell us about the future of cardiac xenotransplantation?
Dr. Moazami: A lot of work needs to be done before we can determine whether this approach can solve the organ shortage. But the time has come for us to do that work. We’ve never been as close to making xenotransplantation a reality as we are now.
Disclosures
Research support and funding for this study was provided by Lung Biotechnology, a wholly owned subsidiary of the United Therapeutics Corporation.