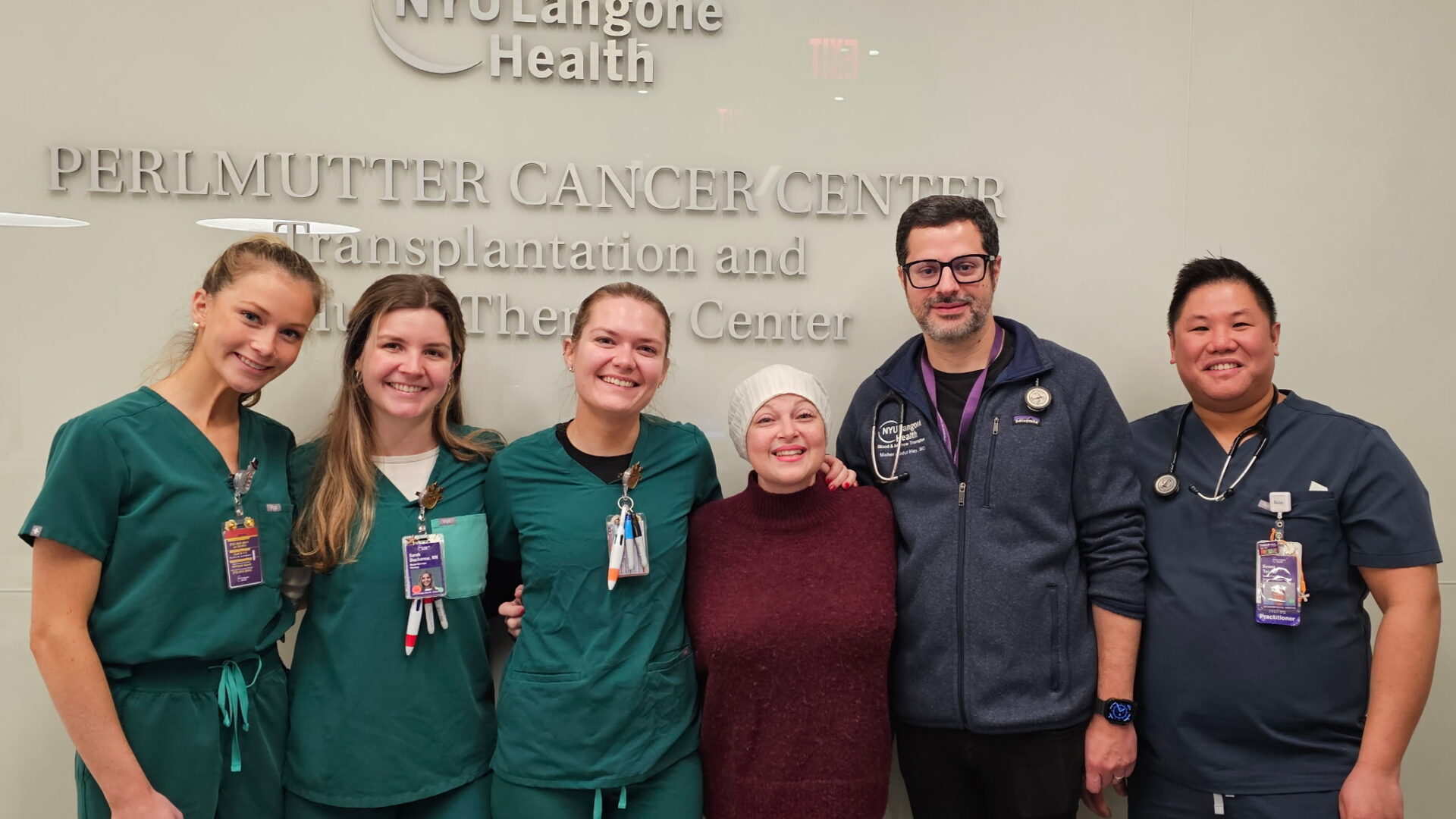Referral Notes:
- A patient with AML has achieved sustained remission after a third allogeneic transplant with sequential menin inhibition (bleximenib) through a clinical trial.
- She continues on post-transplant maintenance bleximenib to prevent relapse.
- NYU Langone’s Blood and Marrow Transplantation and Cellular Therapy Center reports a one-year allogeneic transplant survival rate of over 80 percent and completed its 1,000th stem cell transplant for hematologic malignancy in November 2025.
In January 2025, a woman in her early 40s with acute myeloid leukemia (AML) harboring a KMT2A translocation was referred to Perlmutter Cancer Center hematologist Mohammad Maher Abdul Hay, MD, for a third opinion.
Over five years, she had relapsed despite multiple courses of intensive targeted chemotherapy, two allogeneic cord blood transplants in the absence of a matched donor, and participation in a clinical trial of revumenib—a menin inhibitor that has since received FDA approval for KMT2A translocation. Her initial care team had exhausted its treatment options, and a second institution recommended hospice.
At Perlmutter Cancer Center, however, she wasn’t out of options. Dr. Abdul Hay, serving as site principal investigator, enrolled the patient in a clinical trial evaluating bleximenib, a selective menin inhibitor, for patients with relapsed/refractory or newly diagnosed AML with KMT2A rearrangements.
After she achieved remission on bleximenib, Dr. Abdul Hay pursued a high-risk third allogeneic transplant, reserved for carefully selected patients. The transplant used a haploidentical (half-matched) donor—the patient’s teenage daughter—followed by NYU Langone-developed post-transplant protocols designed to reduce the risk of rejection. The patient was then restarted on bleximenib as maintenance therapy to prevent relapse. “When we believe we can change outcomes for patients, we will take risks,” Dr. Abdul Hay says.
“When we believe we can change outcomes for patients, we will take risks.”
Mohammad Maher Abdul Hay, MD
Ten months post-transplant, the patient remains in remission. “She’s not just in remission. The mutation is undetected, so we know she is in deep remission,” Dr. Abdul Hay adds. Notably, she was the first patient in the world to be enrolled in a study of maintenance bleximenib after allogeneic transplant for AML with KMT2A rearrangement.
The case exemplifies the clinical and investigational expertise of NYU Langone Health’s Clinical Leukemia Program and Blood and Marrow Transplantation and Cellular Therapy Center, which manages a high volume of complex and high-risk referrals.
High Volume, High Standards
Accredited by the Foundation for the Accreditation of Cellular Therapy for adult and pediatric autologous and allogeneic transplant and cell therapy, the comprehensive Blood and Marrow Transplantation and Cellular Therapy Center has made tremendous advances since its inception in 2017.
Directed by Dr. Abdul Hay, the center completed its 1,000th stem cell transplant for hematologic malignancy in November 2025. The center also performed 229 transplants and cellular therapies in 2025, the most yet for the center in a calendar year, with patient volume increasing 20 to 30 percent annually. “We’re expanding enormously, but our growth isn’t compromising quality,” Dr. Abdul Hay says.
“We’re expanding enormously, but our growth isn’t compromising quality.”
According to the Center for International Blood and Marrow Transplant Research, the center’s one-year survival for allogeneic stem cell transplant is 81.7 percent. “We have the best outcomes of any institution in the tristate area,” Dr. Abdul Hay says. “Ours is the only program in the area with a one-year allogeneic transplant survival above 80 percent and has been consistently for the past five years.”
Rates of acute graft-versus-host disease grades 3–4 within 100 days of allogeneic bone marrow transplant are less than 1 percent, compared to approximately 15 percent nationally. “This is a reflection of the prevention protocols we’ve pioneered over the past five years,” Dr. Abdul Hay says.
The center’s emphasis on haploidentical transplantation to expand access for patients underrepresented in donor registries is another key driver of outcomes. “This innovation dramatically increases donor availability,” Dr. Abdul Hay says.
Its success is also supported by novel treatment protocols, including post-transplant chemotherapy, optimized immunosuppression to reduce rejection, and the use of targeted therapies that allow patients to receive lower-toxicity pre-transplant chemotherapy and be in better condition to tolerate transplant.
A Culture of Collaboration
The multidisciplinary team includes transplant physicians, inpatient and outpatient nurses and nurse practitioners, a social worker, pharmacists, apheresis nurses, stem cell laboratory members, the quality and research team, medical assistants, and a nutritionist.
“We are a true team and have immense trust in each other,” Dr. Abdul Hay says. “That leads to better outcomes more than anything else.”
The center currently has 21 active clinical trials underway and is expanding its cellular therapy portfolio, including studies in autoimmune disease. The present case—demonstrating sustained remission with sequential menin inhibition before and after transplant—offers early evidence supporting further study of this approach in AML with KMT2A rearrangement.
“Others thought that if one menin inhibitor didn’t work, a different version wouldn’t either,” Dr. Abdul Hay says. “But here, we proved you can salvage a patient on a different menin inhibitor.”
The plan is for the patient to continue on a maintenance dose for two years before deciding next steps.