A clearer understanding of how a patient’s renal function is likely to change after partial versus radical nephrectomy can be pivotal when helping them choose the surgical approach that best aligns with their goals. Having a realistic estimate of postoperative renal function—and a sense of who is at higher risk for significant decline in renal function—also allows the tailoring of perioperative planning to optimize outcomes.
To fill this gap, our group developed RFAN-ML1, a Machine Learning-based model to estimate Renal Function After Nephrectomy. With just three preoperative variables—age, body mass index (BMI), and baseline estimated glomerular filtration rate (eGFR)—the model provides an individualized estimate of a patient’s expected new baseline renal function following surgery.
To illustrate its usefulness, we describe here how RFAN-ML helped guide a patient who was weighing the trade-offs between a partial and a radical nephrectomy for treatment of an intermediate-sized renal mass.
Case Highlights:
- The patient had a renal mass suspicious for malignancy and was deciding whether the potential for preserved renal function with a partial nephrectomy was worth undergoing the higher risks of complications.
- Her baseline eGFR was 93 mL/min.
- RFAN-ML estimated her postoperative eGFR as 84 ml/min with a partial nephrectomy versus 58 ml/min after a radical nephrectomy, suggesting better long-term kidney and cardiovascular health.
- With a clearer sense of the expected benefit, she confidently chose a partial nephrectomy.
Patient Case
A female in her 70s was found to have a 4.5 cm incidental right, posterior, medial, hilar, endophytic, renal mass on imaging performed for abdominal pain. Her past medical history was notable for stroke, atrial fibrillation, dyslipidemia, arthritis, glaucoma, and she had an eGFR of 93 mL/min. She was on apixaban, gabapentin, and a statin at baseline.
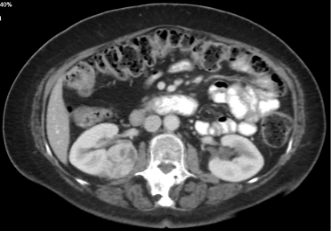
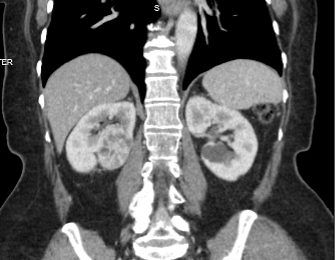
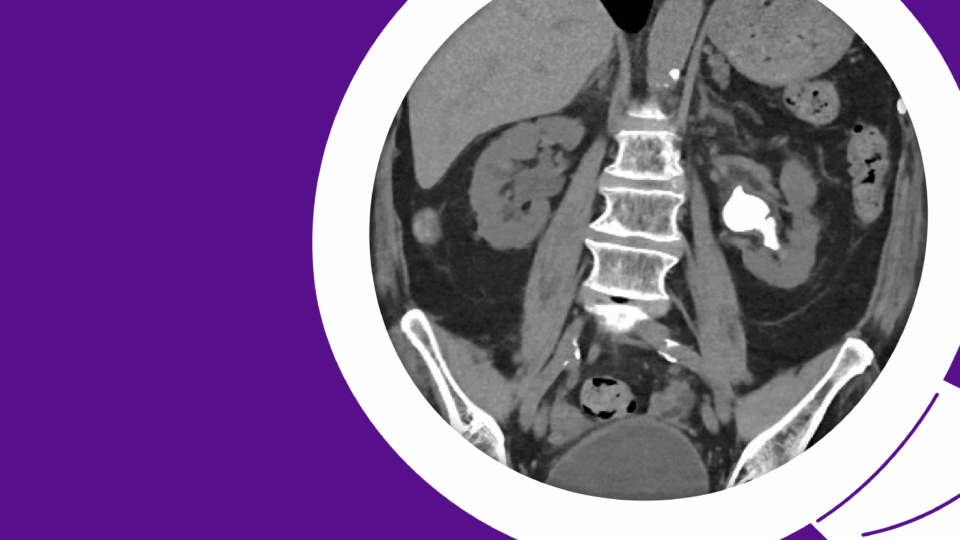
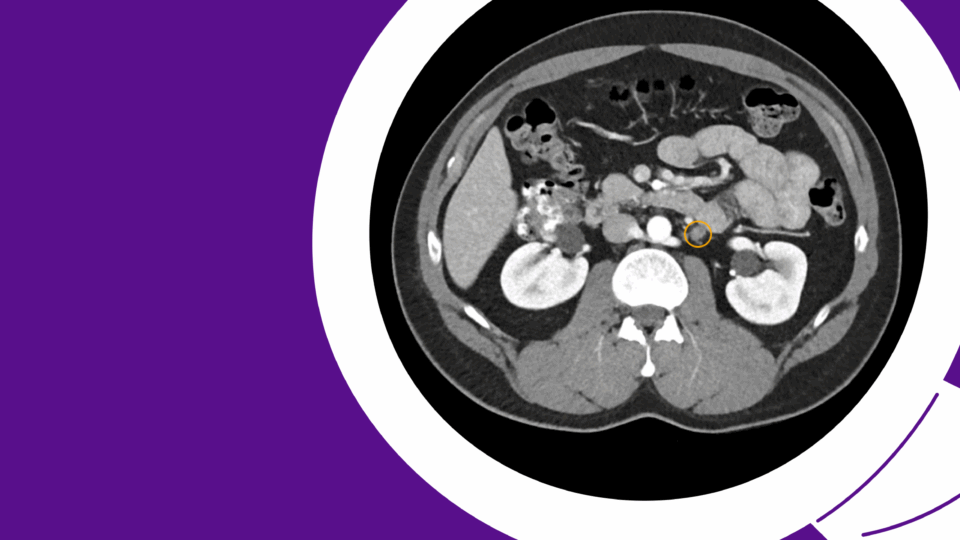
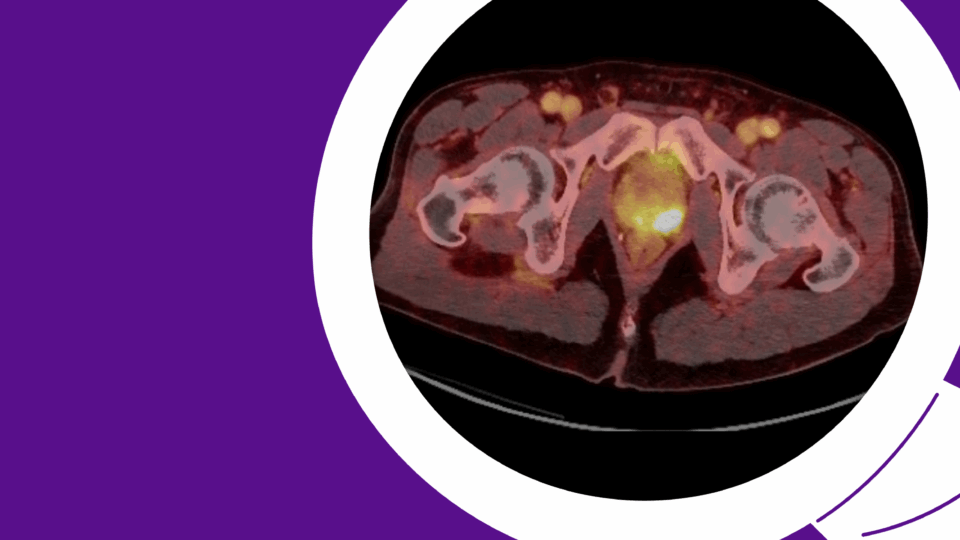
Figure 1. A 4.5 cm right, posterior, medial, renal mass suspicious for renal cell carcinoma. Source: NYU Langone Health.
Based on the appearance of imaging (Figure 1), she was advised that this mass most likely represents a malignancy and the standard treatment is surgical removal. This can be done either through partial nephrectomy, in which the tumor and a portion of surrounding tissue is removed while the remaining, ipsilateral kidney is preserved, or radical nephrectomy in which the entire tumor-bearing kidney is removed.
We discussed the advantage of partial nephrectomy in preserving renal function. However, partial nephrectomy carries higher risks of complications, such as bleeding, urinary leak, and secondary interventions, compared to radical nephrectomy.2,3,4,5 Our patient understood these trade-offs.
She was particularly concerned about how much kidney function would be compromised with a radical nephrectomy versus how much could be saved with a partial. “Is the extra risk of a partial nephrectomy worth it for me?” she asked.
To answer this question with objective data, we turned to the RFAN-ML, a tool which provides patient-specific estimates of postoperative renal function and can inform such shared decision-making discussions.
RFAN-ML
Accurately estimating the renal function after partial or radical nephrectomy can allow for personalized counselling and tailoring of the surgical approach to optimize outcomes for each patient. Furthermore, a preoperative assessment of the estimated postoperative renal function can facilitate earlier identification of patients at high risk of developing a significant decline in renal function, enabling tailored perioperative management to optimize outcomes.
Existing prediction models for estimating renal function after partial or radical nephrectomy have several limitations, including the requirement for detailed clinical information that may not be routinely available. In addition, few models have undergone external validation, and even fewer have been translated into deployed, clinically-usable models.6
To address this gap, our team has developed, validated, and deployed RFAN-ML.1 This model uses only three routinely available, preoperative features—age, BMI, and eGFR—to estimate the new baseline renal function after either partial or radical nephrectomy.
Leveraging the NYU Urology Datahub, we tested the model on data from 891 patients that underwent partial or radical nephrectomy at NYU Langone Health between 2013 and 2023 and found that RFAN-ML demonstrated strong performance.
The patient’s data were input into RFAN-ML (Figure 2), which estimated that the eGFR after partial nephrectomy would be 84 ml/min verus 58 ml/min after radical nephrectomy. We explained that preserving this amount of renal function may translate to better overall health, given the links between kidney function and cardiovascular outcomes.7
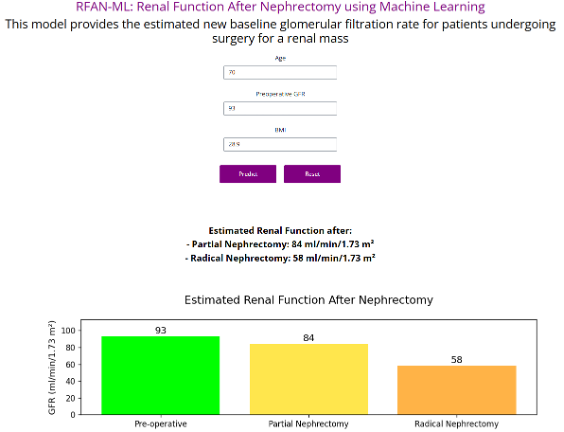
Figure 2. Deployment of RFAN-ML, a simple application to estimate renal function after partial or radical nephrectomy. Source: NYU Langone Health
With a better understanding of the magnitude of the benefit of partial nephrectomy on renal function, the patient elected for this approach. She underwent a successful partial nephrectomy.
Conclusion
The increasing availability of comprehensive data and the growing computational power to process it have fueled the growth in the applications of machine learning and artificial intelligence (AI) in healthcare, including urology. My research group is dedicated to improving outcomes and optimizing resource utilization in urologic oncology using data and advanced analytics.
As director of the NYU Urology Datahub, I oversee a repository of EMR and digital data that is used for research and the development of AI models. RFAN-ML was developed with the motivation to personalize nephrectomy counseling so that patients can make more informed decisions about their care.
Research related to RFAN-ML was presented at the 2024 Society of Urologic Oncology Annual Meeting and the 2025 Machine Learning for Health Care conference, and a full publication is available from JCO Clinical Cancer Informatics.
References
- Persily J, et al. JCO Clin Cancer Inform. 2025;9:e2500086. DOI.
- Stephenson AJ, et al. J Urol. 2004;171(1):130-134. DOI.
- Lowrance WT, et al. J Urol. 2010;183(5):1725-1730. DOI.
- Houjaij A, et al. JU Open Plus 2(5):e00042, May 2024. DOI.
- An JY, et al. Urology. 2017;100:151-157. DOI.
- Pecoraro A, et al. Eur Urol Oncol. 2023;6(2):137-147. DOI.
- Go AS, et al. N Engl J Med. 2004;351(13):1296-1305. DOI.