Referral Notes:
- BB-301 is an experimental gene therapy for OPMD, a rare disorder characterized by weakness of the eyelid and throat muscles; NYU Langone is leading a single-center Phase 1b/2a trial to evaluate its safety and efficacy.
- At present, 2 patients have been treated with a third enrolled; another 21 who have participated in a natural history study will soon be eligible to enroll.
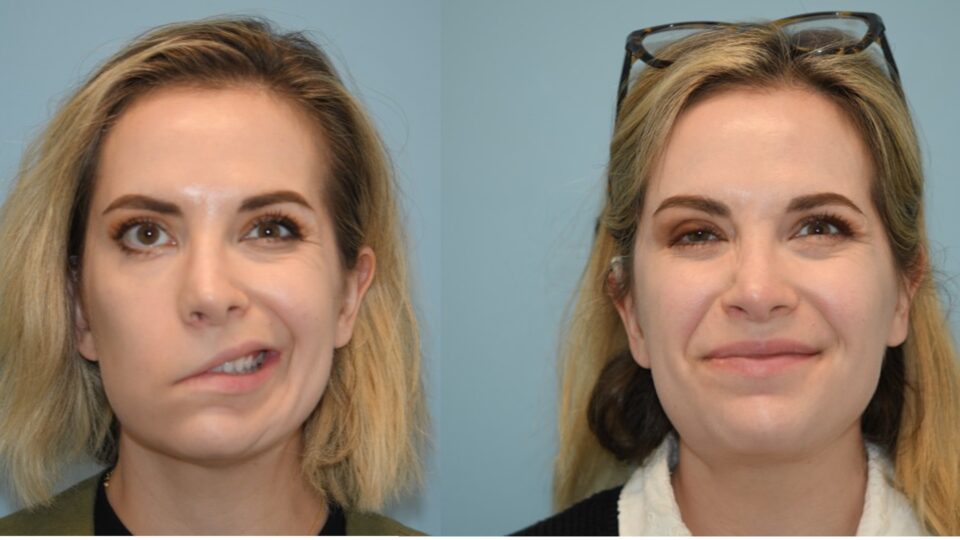
- After 9 months of treatment for the first patient and 6 months for the second, preliminary data show sustained improvements in swallowing.
- Additional interim results are expected in early 2025.
Oculopharyngeal muscular dystrophy (OPMD) is a rare genetic disorder that progressively weakens the muscles of the eyelids and throat. Symptom onset typically begins after age 40, with patients often developing severe dysphagia that can lead to malnutrition, dehydration, and aspiration pneumonia—and, in some cases, death. Although treatments such as cricopharyngeal dilation or myotomy can improve swallowing-related symptoms, relief is usually temporary.
To date, no treatment has been approved that addresses OPMD’s underlying pathology. However, an experimental gene therapy known as BB-301 aims to do just that. A Phase 1b/2a clinical trial began in November 2023 at NYU Langone Health, and preliminary data are encouraging.
“The hope is that this will be a permanent fix for folks with OPMD,” says Milan R. Amin, MD, chief of the Division of Laryngology, who is leading the trial. “These patients know their fate, because they’ve watched family members go through exactly the same thing,” Dr. Amin observes. “As the disease progresses, they’re unable to eat with friends. Many end up on feeding tubes. They’re worried about passing the gene on to their children.”
Silencing and Replacing an Errant Gene
OPMD is caused by mutations in a gene that encodes PABPN1, a protein crucial for RNA processing in cells throughout the body. Clumps of abnormal PABPN1 accumulate in some muscle cells, impairing their function and eventually killing them.
BB-301 is designed to stop this cascade of destruction. Developed by the California-based Benitec Biopharma, the therapy uses an adenovirus vector to deliver genetic code that silences the dysfunctional PABPN1 gene and replaces it with a functional version. The vector is injected into the pharyngeal constrictor muscles through incisions on both sides of the neck. “The open approach enables us to deliver the therapy directly to the muscles, which has been shown preclinically to generate a stronger gene effect,” Dr. Amin explains.
“Right now, we’re just looking at safety, but we’ve begun seeing some potential efficacy as well.”
Milan R. Amin, MD
The single-center study’s Phase 1b phase will test three escalating doses of the therapy, leading to a Phase 2a trial that will test the optimal dose. Two patients have been treated so far, with a third patient recently enrolled. An additional 21 patients who have participated in a natural history study will soon be eligible to enroll.
“Right now, we’re just looking at safety,” Dr. Amin says, “but we’ve begun seeing some potential efficacy as well.”
Offering Patients New Hope
To measure BB-301’s effects on swallowing ability, the researchers assess total pharyngeal residue using four types of food: a thin liquid, a moderate liquid, a thick liquid, and a solid. Early results have been released for the first patient, with positive responses seen at 90 and 180 days of follow-up.
For moderate and thick liquids, as well as solid foods, pharyngeal residue was lower than at any point during a previous natural history study of the patient. For thin liquid, residue was comparable to the lowest value seen during that study. The patient’s self-reported swallowing difficulties have also improved, and neither she nor the other participant treated have experienced major adverse effects.
The second patient has also shown improvements at 90 days of follow-up, according to Dr. Amin, though her presenting symptoms were milder, and their mitigation has been less dramatic.
“This treatment has had an impact [on the first patient] beyond just her physical health. She told me, ‘I now have hope.’”
Much more research will be needed to answer the questions raised by these findings, such as whether BB-301 brings improvements to a sizable proportion of patients, and whether (as with Elevidys, the gene therapy recently approved for Duchenne muscular dystrophy) a single dose can elicit durable effects. Additional preliminary results are expected in early 2025.
For patients with OPMD, however, the trial already offers a different kind of benefit—a sense of possibility. As Dr. Amin puts it, “If it can reverse their dysfunction, it may significantly improve not only their quality of life but also their lifespan.”
The trial’s first subject, he adds, has resumed going to restaurants, no longer fearing that others will see her struggling to swallow. “This treatment has had an impact beyond just her physical health,” Dr. Amin says. “She told me, ‘I now have hope.’”
Disclosures
NYU Langone Health receives funding from Benitec Biopharma to support the Phase 1b/2a trial of BB-301 for OPMD.