The management of localized prostate cancer has evolved in many ways over the past 20 years. The diagnostic paradigm has shifted, owing to the availability of alternative biomarkers to PSA and imaging to better assess the risk of prostate cancer.
In particular, MRI has served as a means of assessing risk and guiding biopsies, thereby improving disease detection through better identification of high-risk populations and more accurate sampling of the prostate. The Department of Urology at NYU Langone Health has played a leading role in guiding this diagnostic paradigm shift through characterization and refinement of pre-biopsy MRI and MRI-targeted (fusion) biopsy techniques.1,2,3 At our center, since 2012, all men presenting with suspicion of prostate cancer have undergone prostate MRI for risk assessment and subsequent guidance of necessary biopsies.4 Such efforts have greatly improved diagnostic accuracy, patient counseling, and the appropriate use of treatments when necessary.
Upon diagnosis, the recognition that not all prostate cancers threaten mortality—and that deferring initial treatment is not harmful for many patients—has necessitated careful patient counseling to enable informed, shared decision-making. Moreover, the addition of imaging and image-targeted biopsy to the diagnostic armamentarium has opened the door to targeted partial gland treatments or focal therapy for patient consideration. Technological advances have also evolved techniques in radiation and surgery, affecting both short- and long-term risks. As such, the decision-making process can be complex for both patients and physicians.
Here, we present a case of a young male with intermediate-risk prostate cancer. We detail our stepwise shared decision-making process that helped the patient elect radical prostecteomy. We also overview the surgical approach and our same-day discharge protocol and its success.
Referral Notes:
- A 54-year-old male with a family history of prostate cancer presented with rising PSA levels and MRI findings suggested high-risk disease.
- Biopsy confirmed Gleason 3+4 cancer in the left-sided region of interest, along with multifocal Gleason 3+3 disease. Genomic testing revealed a Decipher score of 0.75.
- After counseling on treatment options, he chose robotics-assisted radical prostatectomy; surgery was successful with same-day discharge.
- Postoperatively, he regained continence within two weeks, reported adequate erectile function, and achieved undetectable PSA levels (<0.01 ng/mL) through nine months of follow-up.
Patient Case
A 54-year-old male with a family history of prostate cancer presented with a rising PSA level, increasing from 2.51 in January 2022 to 7.66 ng/mL in May 2024.
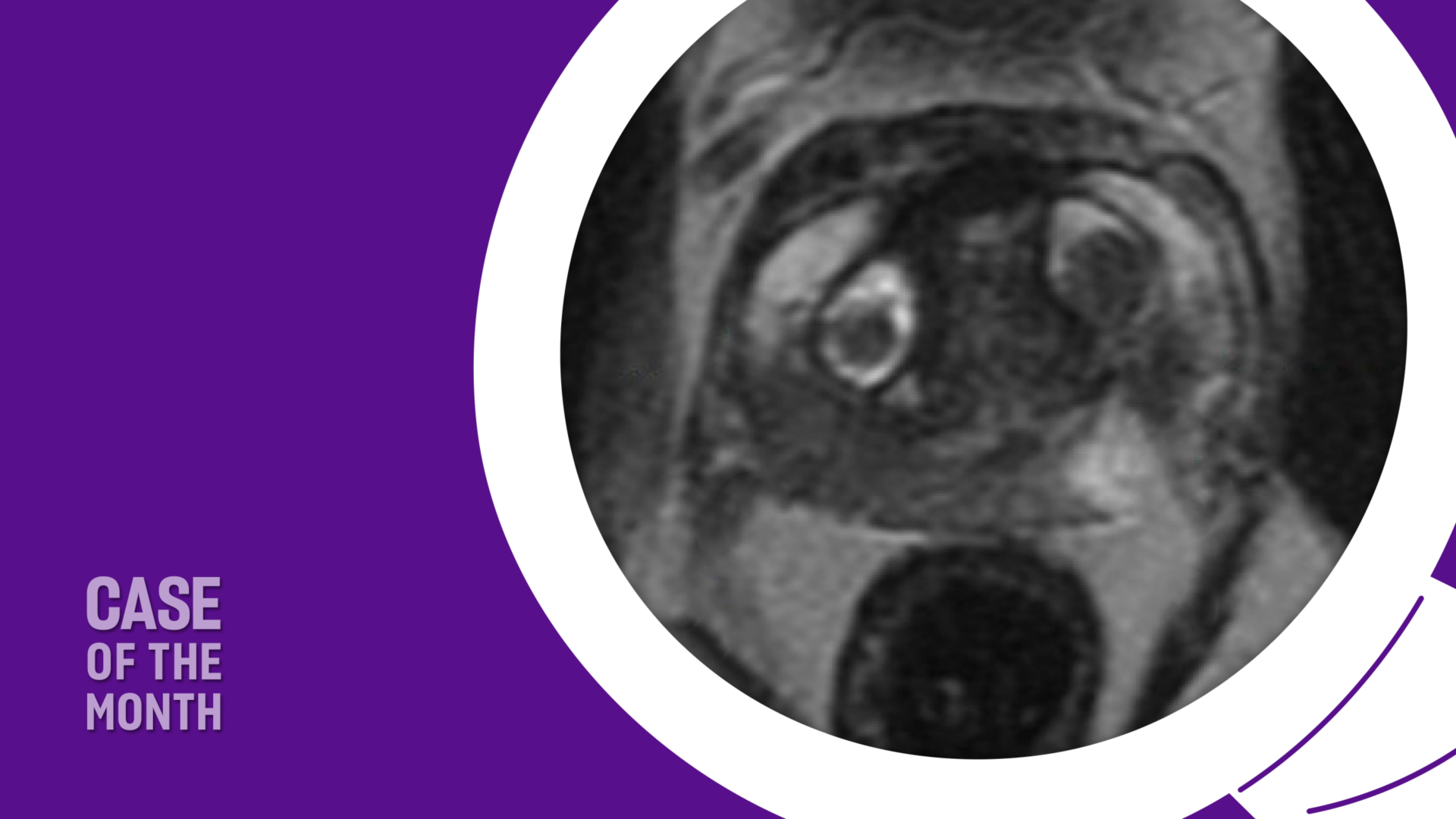
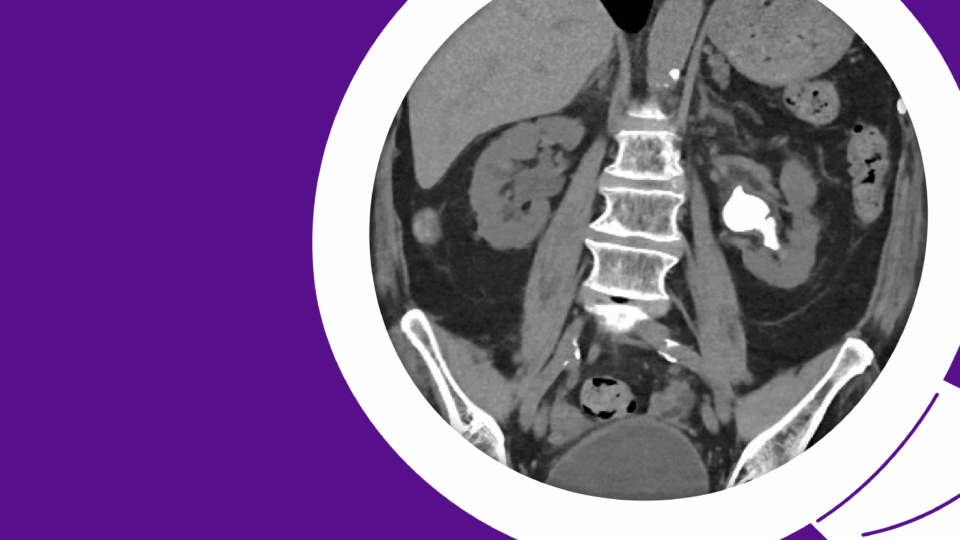
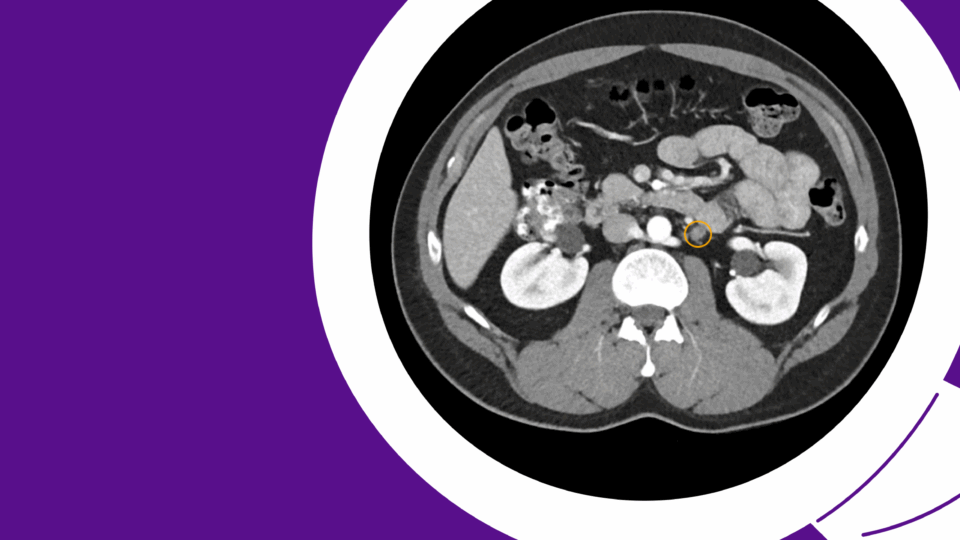
A prostate MRI revealed a 41 cc gland (PSA density = 0.19), a PI-RADS 4 abnormality in the left posterolateral midgland peripheral zone, and a PI-RADS 3 abnormality in the right posterolateral midgland peripheral zone (Figure 1). A prostate biopsy demonstrated Gleason 3+4 cancer in the left-sided region of interest and systematic cores, as well as Gleason 3+3 cancer in the right-sided region of interest, along with multifocal Gleason 3+3 cancer in systematic cores. Genomic testing revealed a Decipher score of 0.75, suggesting high risk.

Upon presenting to NYU Langone Health, he was counseled regarding options using a stepwise counseling methodology as part of the shared decision-making process. Options—including active surveillance, partial gland ablation (focal therapy), radical prostatectomy, and radiation—were reviewed sequentially (Figure 2).
| Step | Key Considerations |
|---|---|
| Step 1: Surveillance Consideration | Balance of disease risk against longevity over time Consideration of patient compliance with testing Consideration of patient preferences and priorities |
| Treatment Step 2: Consideration of Partial Gland Treatment/Focal Therapy | Evaluate disease risk, focality, location, size Educate patient regarding risks and benefits Consideration of patient compliance, strategies for salvage |
| Whole Gland Treatment Decision 3: Surgery vs. Radiation Whole Gland Treatment | Expected outcomes and potential toxicities Impact on current QOL Risk of recurrence/secondary treatment over time |
| Decision 4: Specific Considerations of Treatment | Surgery: Robotic vs. open, lymph node dissection, choice of surgeon Radiation: External vs. interstitial, fractionation, androgen deprivation |
His medical history was significant only for previous endocarditis requiring mitral valve repair. His father previously underwent radical prostatectomy, without evidence of recurrence. His baseline urinary and sexual function were normal, with an IPSS score of 0 and a SHIM score of 25.
Upon reviewing his personal priorities and preferences, he elected to undergo robotics-assisted radical prostatectomy. The patient was enrolled in the NYU Langone same-day discharge protocol, with the intention of discharging him from the recovery room after surgery. As part of this protocol, care and recovery expectations were outlined in the preoperative education meeting.
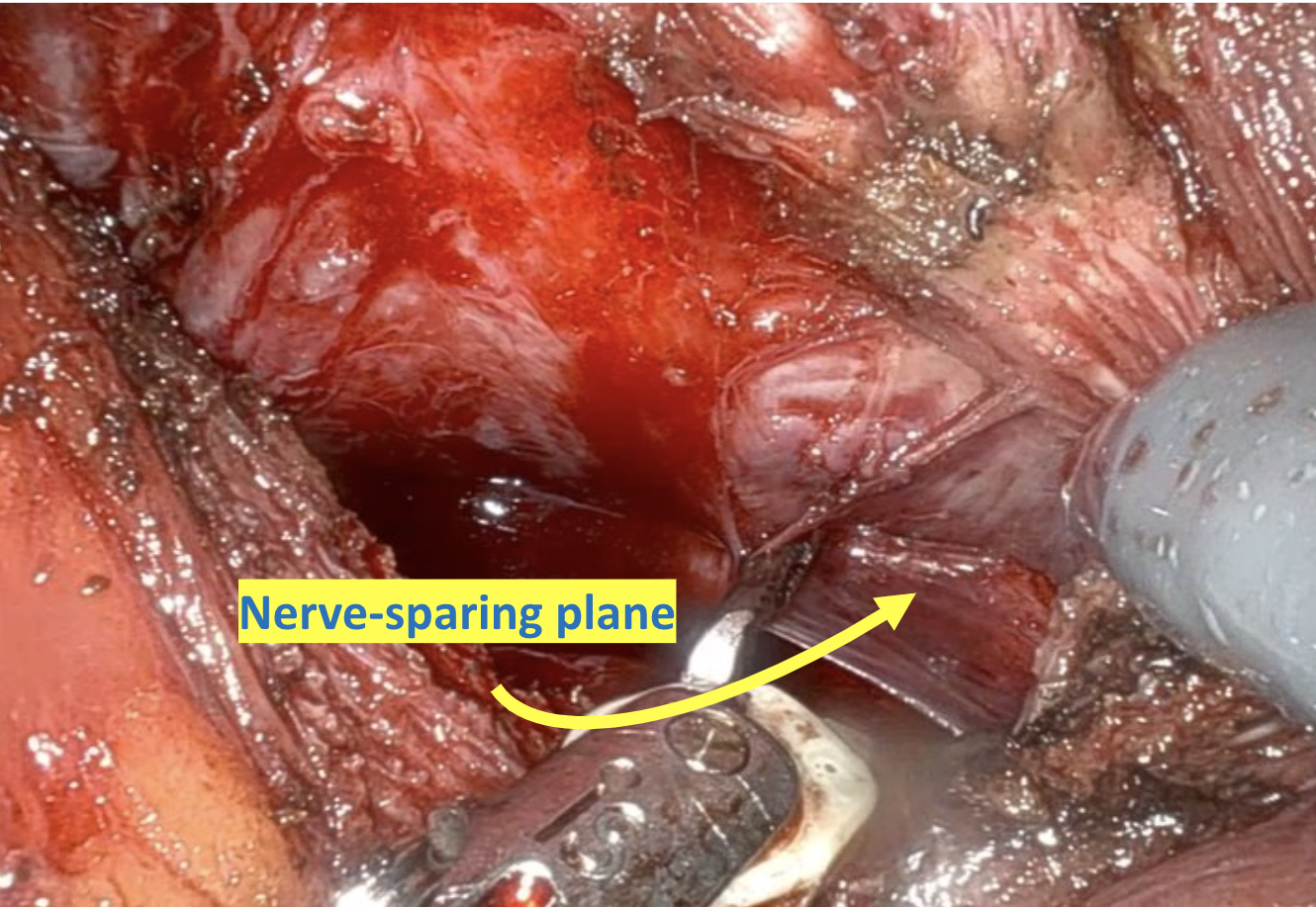
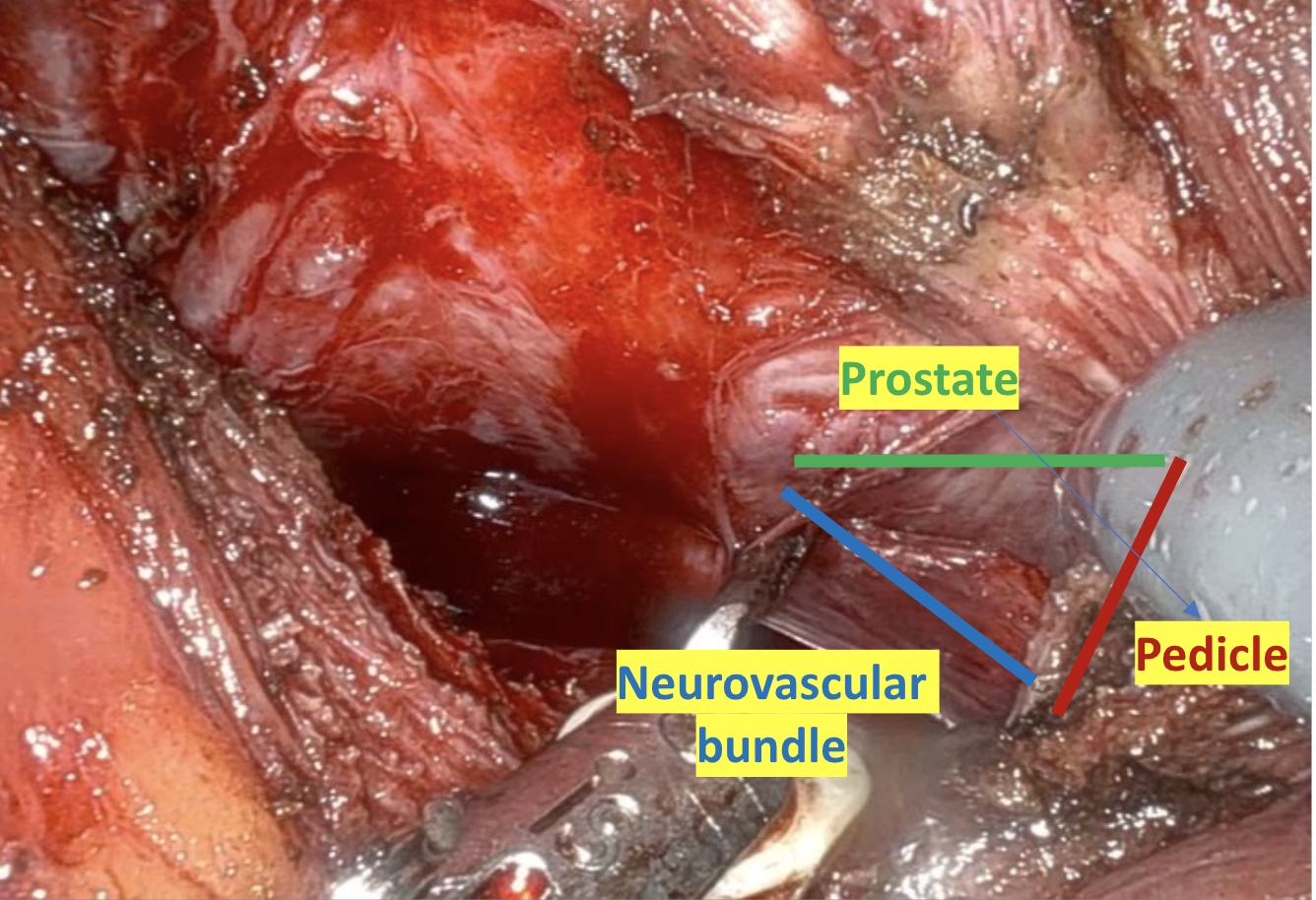
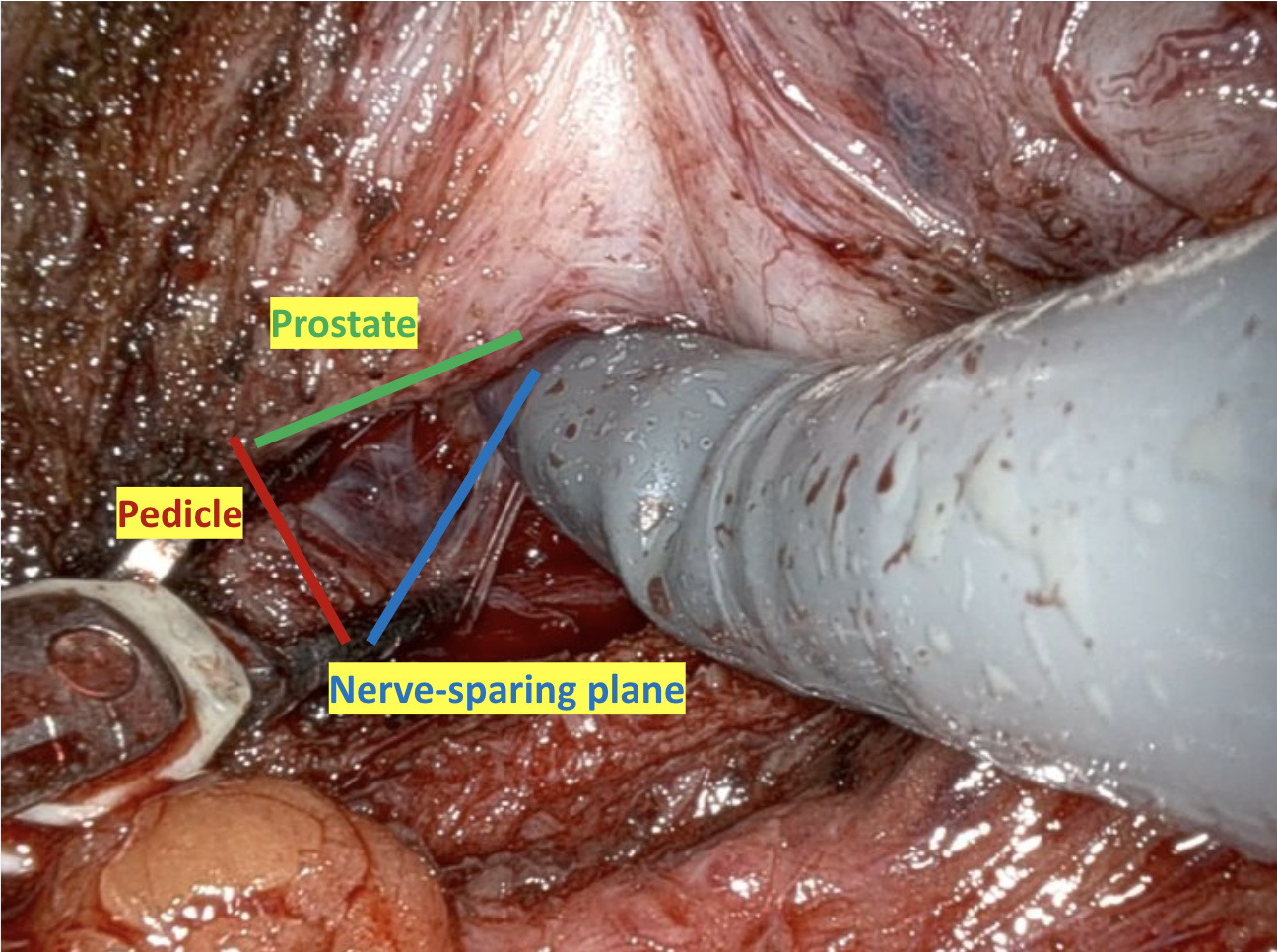
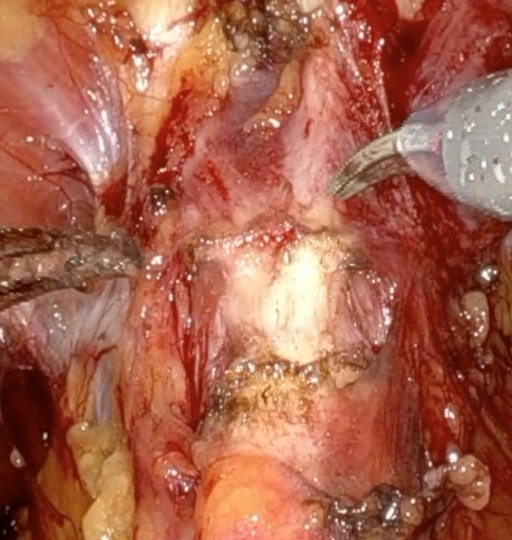
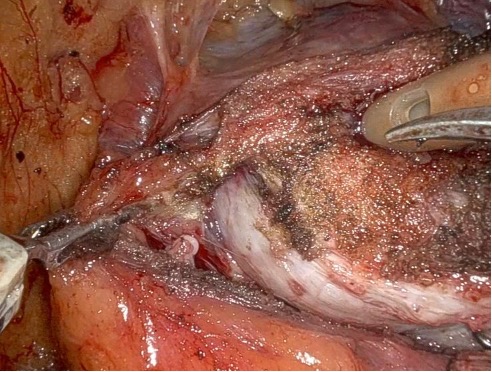
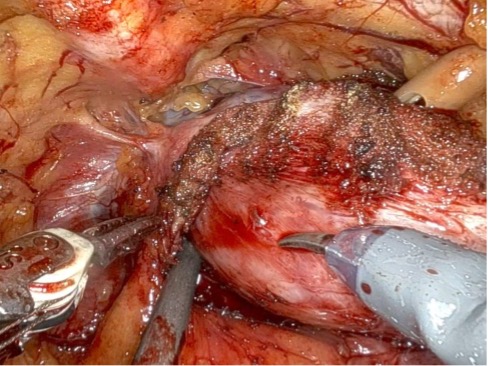
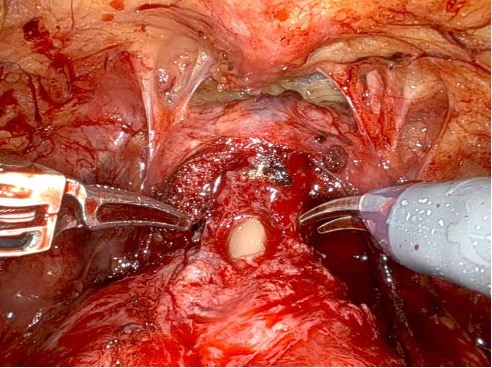
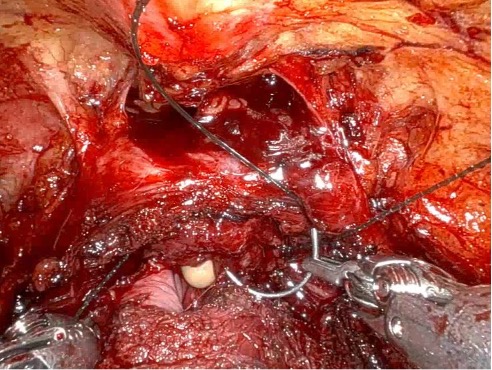
Given the patient’s disease risk, cancer location, and volume, an anterior approach transperitoneal bilateral nerve-sparing procedure (Figure 3,4), with preservation of the anterior and lateral overlying fascia (Figures 5–9), was performed. Surgery was conducted at a low insufflation pressure of 5 mm Hg to reduce postoperative pain and enhance confidence in postoperative hemostasis. The case was uncomplicated, with an estimated blood loss of 100 cc. At completion, ketorolac was administered along with an intravenous fluid bolus of 1,000 cc normal saline. In the recovery room, he was aggressively hydrated. Upon waking, he was encouraged to drink fluids and ambulate. As his vital signs and urine output remained favorable, he was discharged from the recovery room three hours after surgery, following a check of postoperative labs.
His pathology revealed Gleason 3+4, pT3a, N0, Mx prostate cancer with negative surgical margins. No adjuvant therapy was recommended. He regained total continence within two weeks. At the three-month postoperative visit, he was not using incontinence pads or diapers. He reported full erections adequate for intercourse, though of shorter duration than preoperatively, with the use of oral phosphodiesterase type 5 inhibitors. His PSA declined to undetectable (<0.01 ng/mL) and has remained undetectable through nine months postoperatively.
Four Steps in Shared Decision-Making
In this case, a stepwise decision process, previously developed in the Department of Urology at NYU Langone Health,5 was applied to simplify information processing and guide the patient thematically through the most essential concepts in risk-based care (Figure 2).
Step One: Active Surveillance Consideration The risks and benefits of active surveillance were discussed. Contemporary comparative data suggest that initial surveillance is safe for most men with favorable-risk disease, provided they comply with follow-up. The randomized ProtecT trial found no increase in disease-specific mortality when initial surveillance was applied, though approximately 60 percent of men required delayed treatment, and metastasis risk appeared increased in this non-selected population.6 Factors predicting increased risk of progression or reclassification in this patient included a high PSA density, a high genomic risk score, and a high-suspicion MRI score. Given these risks, he elected not to pursue initial surveillance.
Step Two: Partial Gland Ablation Consideration Given his disease risk, volume, imaging characteristics, and biopsy concordance, partial gland ablation (focal therapy) was considered. However, the presence of multifocal Gleason 3+3 disease and a PI-RADS 4 abnormality on the right suggested that complete ablation of all known disease was not feasible without whole-gland treatment. The patient declined focal therapy.
Step Three: Whole Gland Treatment Consideration The risks and benefits of radiation therapy and radical prostatectomy were reviewed, including potential side effects, complications, and long-term efficacy. Given his young age and normal baseline potency and urinary function, discussions emphasized the risks of erectile dysfunction, urinary incontinence, and, in the case of radiation, bowel toxicity. The patient was offered a consultation with radiation oncology. Ultimately, after weighing data presentation with personal priorities, he opted for radical prostatectomy.
Step Four: Surgical Approach Selection The majority of contemporary patients elect robotic-assisted radical prostatectomy (RARP) due to reduced blood loss, faster convalescence, and lower perioperative complication rates compared with open surgery. The patient proceeded with RARP.
Surgical Approach
At NYU Langone Health, efforts have been made to improve RARP outcomes, including techniques for anterior fascia preservation, which may facilitate early return of urinary continence.7,8 Two approaches to anterior fascia preservation include the posterior (“Retzius-sparing”) approach, in which the prostate is removed entirely through an exposure posterior to the bladder in the pre-rectal space, and the anterior approach, in which the bladder is dropped from the anterior abdominal wall and the prostate is removed through the space of Retzius from beneath the anterior fascia after division of the bladder neck.
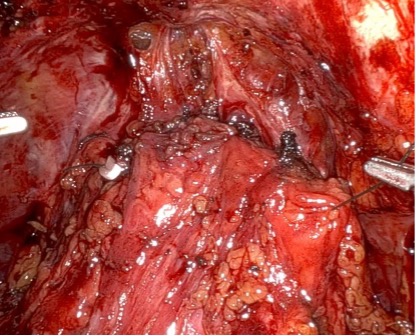
In this case, an anterior approach was utilized, in which the bladder neck division is carried out by dividing the outer detrusor fibers cranially, away from the prostate, to allow formation of a detrusor apron along the endopelvic fascia (Figure 5). The bladder neck is then developed by elongating the inner detrusor in a telescope fashion prior to division. Following seminal vesicle mobilization, the posterior pre-rectal plane behind the prostate is developed to expose the base of the gland. Nerve-sparing is initiated by identifying the posterolateral base of the gland and developing a “triangle dissection” bordered by the base of the prostate, the prostate pedicle, and the underlying neurovascular bundle as it courses beneath the pedicle (Figure 3, 4). Upon separating the neurovascular bundle from the pedicle, the pedicle can be safely divided to the lateral detrusor attachments of the overlying anterior fascia. Lateral attachments are released, thus allowing the prostate to be slowly lifted out from underneath the fascia, progressively separating the NVB under direct vision posteriorly and preserving a layer of detrusor and connective fascia over the anterior gland to the level of the apex (Figure 6).
Such efforts may improve early return of continence, as seen in this case, though long-term continence outcomes are similar to conventional approaches. Careful evaluation of surgical margins is essential, as dissection closer to the prostate may lead to higher positive margin rates.
Same-Day Discharge Protocol
An accelerated recovery pathway has been implemented at NYU Langone Health to discharge patients on the day of surgery, reducing hospital-acquired infections, promoting earlier ambulation, and enhancing comfort. The protocol includes preoperative education, intraoperative modifications (such as low insufflation pressure and fluid management), and structured postoperative care.
Since initiating the same-day discharge protocol in 2023, approximately 60 percent of patients undergoing RARP at NYU Langone now elect for discharge from the recovery room. The impact of surgical and perioperative modifications on long-term recovery and functional outcomes remains an area of ongoing study.
References
- Mendhiratta N, et al. J Urol. 2015;194(6):1601-1606. DOI.
- Meng X, et al. Eur Urol. 2016;69(3):512-517. DOI.
- Mendhiratta N, et al. Urology. 2015;86(6):1192-1198. DOI.
- Rosenkrantz AB, et al. Urol Int. 2016;97(2):247-248. DOI.
- Taneja SS, Bjurlin MA. Active management strategies in localized prostate cancer. In: Partin AW, Peters CA, Kavoussi LR, Dmochowski RR, Wein AJ, eds. Campbell-Walsh-Wein Urology. 12th ed. Elsevier; 2025. In press.
- Hamdy FC, et al. N Engl J Med. 2023;388(17):1547-1558. DOI.
- Kowalczyk KJ, et al. J Urol. 2021;206(5):1184-1191. DOI.
- Wagaskar VG, et al. Eur Urol. 2021;80(2):213-221. DOI.