Anti-VEGF drugs to treat retinal disease represent a major cost for the healthcare system and for patients. Several such medications are currently approved by the FDA for intraocular use, including aflibercept, ranibizumab and the biosimilars ranibizumab-nuna and ranibizumab-eqm. In addition, a cancer drug, bevacizumab, is widely used off-label and for a relatively low cost due to the ability to repackage one oncologic dose vial into multiple doses for intraocular use.
However, an investigational bevacizumab biosimilar, now awaiting FDA approval, might challenge the use of off-label bevacizumab as an economical alternative.
While biosimilars generally help drive costs down, a study led by NYU Langone Health researchers suggests that this drug—bevacizumab-vikg—could substantially increase the cost burden of intravitreal anti-VEGF agents.
“Unless the FDA can be persuaded to allow an exemption for repackaged bevacizumab, the introduction of an intraocular bevacizumab biosimilar could raise costs dramatically.”
Ravi Parikh, MD, MPH
That danger arises, the team notes, because the U.S. Drug Quality and Security Act prohibits the compounding of a copy of an approved medication. If the FDA approves a bevacizumab biosimilar for intraocular use, the repackaging of bevacizumab cancer drugs for such purposes could be curtailed. Meanwhile, the biosimilar could command a higher price.
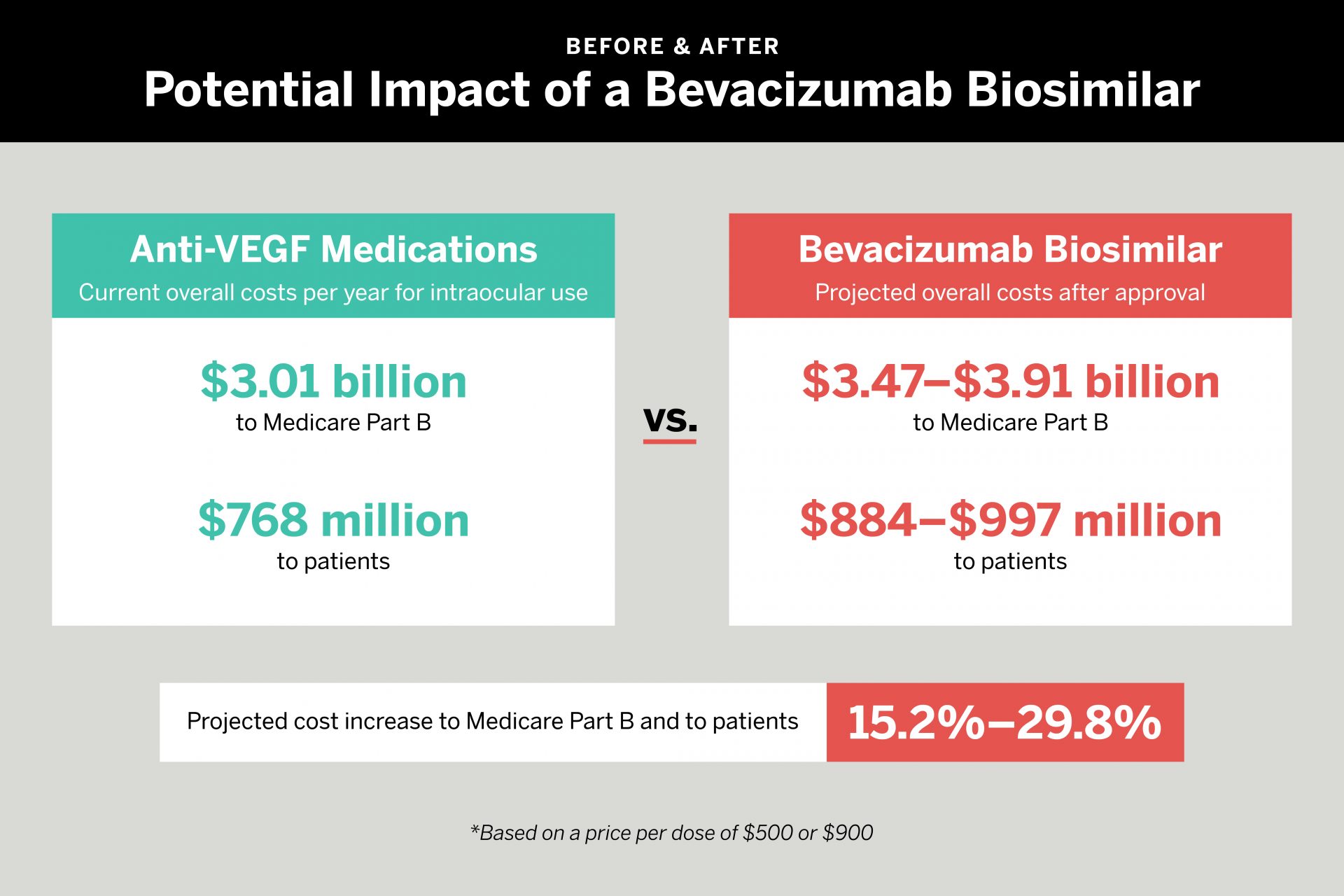
To determine the impact of such a scenario, the researchers calculated Medicare Part B and patient payments (directly or via secondary insurance) for the most commonly used medications for which data is available. Aflibercept was the most expensive option, at a cost per dose of about $1,451 to Medicare and $361 to patients. Repackaged bevacizumab was least expensive, at $70 to Medicare and $18 to patients.
If an intraocular bevacizumab biosimilar was priced at $500, the team found, costs to Medicare would increase by 15.2 percent per year—from $3.01 billion to $3.47 billion. Patient responsibility would increase from $768 million to $884 million. If the new biosimilar was priced at $900, Medicare costs would rise to $3.91 billion, an increase of 29.8 percent. Patient responsibility would soar to $997 million.
“Unless the FDA can be persuaded to allow an exemption for repackaged bevacizumab,” says senior author Ravi Parikh, MD, MPH, director of healthcare delivery research for NYU Langone’s Department of Ophthalmology, “the introduction of an intraocular bevacizumab biosimilar that is approved for intraocular use could raise costs dramatically, and also likely limit access to an anti-VEGF drug that has been demonstrated to be safe and effective for nearly 20 years.”
Disclosure: Dr. Parikh has received consulting fees from Anthem Blue Cross Blue Shield, Apellis Pharmaceuticals, GLC Consultants, and Health and Wellness Partners.