Directly injecting biologics into lung cancer tumors might improve response rates beyond those achievable with systemic therapy alone, according to the research behind a new phase II clinical trial testing the approach.
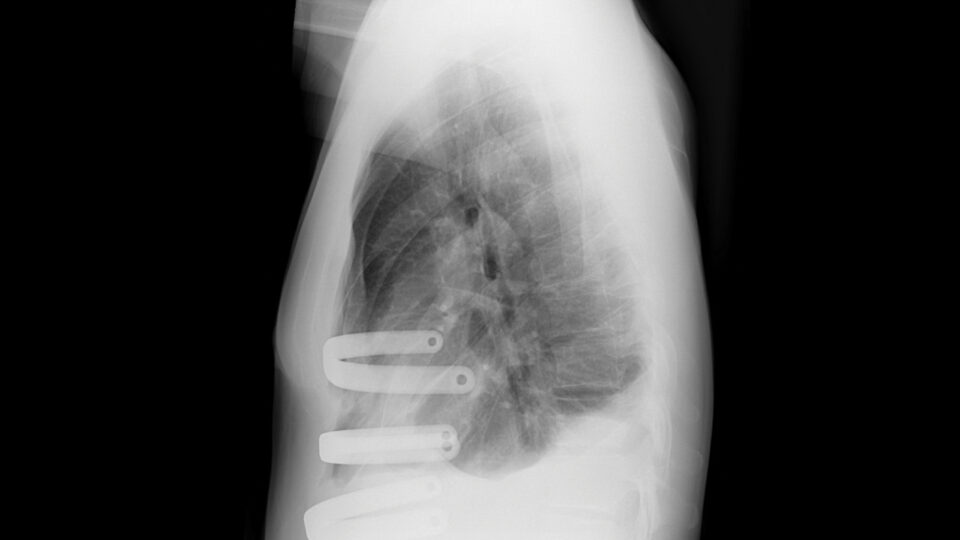
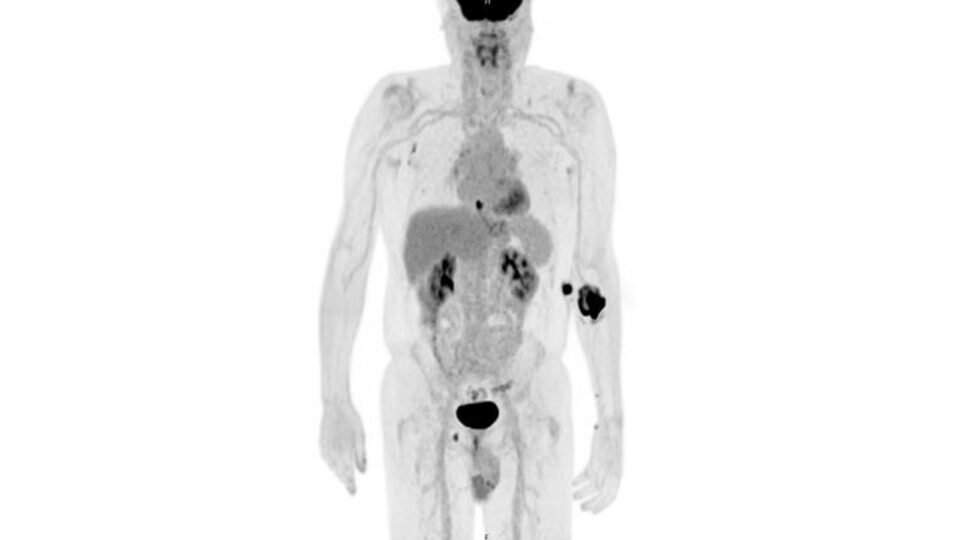
The pioneering open label, multisite trial combines gene-mediated cytotoxic immunotherapy with standard of care for patients with refractory stage III/IV non-small cell lung cancer. The immunotherapy—two courses of aglatimagene besadenovec—is injected directly into an accessible tumor site under CT or ultrasound imaging.
Patients also receive 14 days of oral valacyclovir to heighten broader immune responses to the injectable biologic, which contains herpes simplex virus gene HSVtk.
The two-punch approach directly manipulates the tumor microenvironment while supporting systemic effects, explains principal investigator Daniel H. Sterman, MD, Thomas and Suzanne Murphy Professor of Pulmonary and Critical Care Medicine.
“When you give the patient valacyclovir, the drug in the tumor site is converted into a toxic metabolite that kills tumor cells and activates—together with HSVtk—a robust immune response. It acts not only locally where you do the injection, but anywhere in the body where tumor cells may be.”
“The drug in the tumor site is converted into a toxic metabolite that kills tumor cells and activates—together with HSVtk—a robust immune response.”
Daniel H. Sterman, MD
The study is one of the first to deliver immunotherapy directly into patients’ lung tumors. Patients will be followed for three years following the investigational treatment. Researchers will assess tumor response rates, adverse events, immune cell responses, patient-reported symptoms, and survival rates.