Managing nonobstructive azoospermia (NOA) is the most challenging situation within the field of male infertility. Since 2022, we have offered extended sperm search and microfreeze (ESSM) as a thorough yet noninvasive retrieval option to every patient with NOA prior to considering microsurgical testicular sperm extraction (microTESE). This two-step protocol has increased our sperm identification rate from 40 to 57 percent and allowed many men to forgo invasive procedures.
When ESSM is unsuccessful, microTESE remains the standard of care and enables paternity for many patients. Importantly, starting with ESSM does not affect the success of a subsequent microTESE procedure.
In this Case of the Month, we highlight the successful fertility treatment of a 37-year-old with NOA as demonstration of this effective protocol. Although sperm was not identified through ESSM, microTESE proved successful, underscoring that ESSM can be easily incorporated as a first-line option without impacting the efficacy of microTESE.
Case Highlights:
- The patient presented with testosterone deficiency which we addressed with anastrozole, achieving a eugonadal state for three months before undergoing unsuccessful ESSM.
- MicroTESE identified 660 sperm, resulting in seven embryos through IVF; pregnancy occurred after three embryo transfers.
- Our findings on ESSM’s role in enhancing sperm identification without compromising microTESE success were shared at the 2025 AUA Annual Meeting.
- In our practice, patients see the additional cost of ESSM as justified for the chance to avoid surgery.
Patient Case
A 37-year-old male presented with 12 months of primary infertility and NOA. He and his 37-year-old wife were appropriately timing sexual activity with her ovulation window. He described intermittent mild erectile dysfunction (Sexual Health Inventory for Men 21/25) that he attributed to stress, but this did not interfere with conception attempts.
He has a history of Crohn’s disease which is well managed with ustekinumab, a monoclonal antibiody with no effect on spermatogenesis. He denied smoking or using illicit drugs and has no relevant family history. He is a software engineer and denied genital trauma or exposure to toxic or radioactive agents. His wife has never been pregnant and is otherwise healthy.
Evaluation
The patient had a normal masculine hair distribution. His BMI was 31.7. No gynecomastia was present. Testes bilaterally were soft and about 6 cc (normal volume, ≥ 18 cc). No varicocele was present.
A semen analysis was performed, which was fructose positive with normal volume, normal pH, and no sperm seen after centrifugation. This was repeated with similar results.
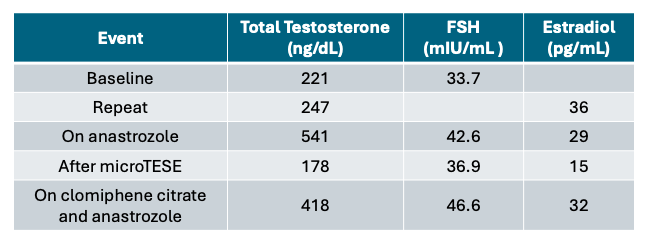
His serum total testosterone was low at 221 ng/dL (normal 300 – 1080 ng/dL) and his follicle stimulating hormone (FSH) was elevated at 33.7 mIU/mL (normal 1.5 – 12.4 mIU/mL) (Table 1).
Given his symptomatic testosterone deficiency and obesity, further work-up confirmed low testosterone at 247 ng/dl, and an estradiol level of 36 pg/mL (normal 7.6 – 42.6 pg/mL) (Table 1). He underwent a genetic evaluation that resulted in no Y chromosome microdeletions detected and a 46,XY karyotype.
Management
His wife was referred to a reproductive endocrinologist, and her evaluation was normal. He was started on anastrozole, with normalization of his testosterone to 541 ng/dL (Table 1). After three months at a eugonadal state, he underwent an extended sperm search, which did not identify any sperm in the ejaculate.
He subsequently underwent microTESE coordinated with an egg retrieval from his wife. During this procedure, 660 sperm were identified. The testis biopsy obtained during this procedure revealed a histologic diagnosis of hypospermatogenesis.
The sperm obtained from the microTESE were used with intracytoplasmic sperm injection on 17 mature eggs, 12 of which fertilized. This IVF cycle resulted in seven embryos which underwent pre-implantation genetic testing, and five of these embryos were euploid.
The patient’s wife became pregnant after three embryo transfers, and delivered a healthy baby.
Three months postoperatively and off anastrozole, the patient’s testosterone was 178 ng/dL. His testosterone deficiency was managed with clomiphene citrate and anastrazole resulting in a testosterone of 418 ng/dL (Table 1).
Discussion
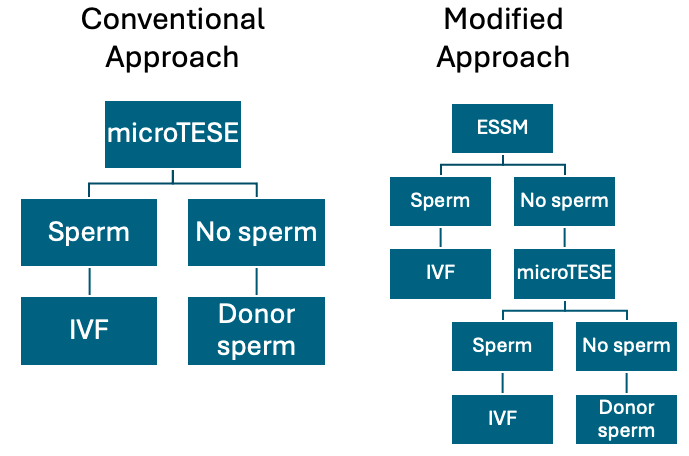
NOA, characterized by the absence of spermatozoa in the ejaculate due to spermatogenesis failure, represents the most severe form of male infertility, affecting approximately 1 percent of men.1 The standard treatment for NOA is microTESE (Figure 1).2 Despite minimized risks with microsurgery, concerns about testicular damage, postoperative testosterone deficiency, and anesthesia exposure remain.3
ESSM offers a noninvasive method for identifying spermatozoa in the ejaculate of men with azoospermia.4 This technique involves partitioning semen samples into microliter droplets and examining each droplet under a high-powered microscope to cryopreserve individual spermatozoa.
As the name implies, the process differs from a standard centrifuged semen analysis to the extent to which a semen sample is evaluated. Some of our patients have had samples evaluated with over five hours of search time.
Since 2022, I have asked every patient with NOA to undergo an ESSM before we move forward with a microTESE (Figure 1). If the ESSM yields sufficient sperm for IVF, then microTESE is not performed.
We presented our experience integrating ESSM into the management of men with NOA at the 2025 American Urologic Association (AUA) Annual Meeting.5 Our experience demonstrates some key advantages with this approach.
Improved Sperm Identification Rates. Prior to the integration of ESSM into our management of men with NOA, we identified sperm in approximately 40 percent of men who underwent microTESE. Our new protocol has resulted in the identification of sperm in 57 percent of men, many of them through ESSM alone. While the risks of microTESE are low, men with a positive ESSM will avoid any risk of iatrogenic testosterone deficiency from a microTESE.
No Impact on MicroTESE Success. The patient in this case unfortunately did not have sperm identified on ESSM. Approximately 60 percent of men with NOA will not have a successful ESSM, and will still require a microTESE procedure. Counterintuitively, the sperm retrieval rates for microTESE have not declined. We still find sperm in 40 percent of men during a microTESE, even after an unsuccessful ESSM.
The primary disadvantage of recommending an ESSM prior to microTESE is cost. Given the extensive time requirements, an ESSM costs approximately ten times more than a standard semen analysis. While an ESSM is more affordable than a microTESE, this approach adds significant cost to the patients who still require microTESE.
Despite the increased potential cost, the significantly improved sperm identification rate (57 vs. 40 percent) has been perceived as justified by patients. Given the added cost, it is important to optimize the patient as much as possible prior to ESSM, as was done with this patient’s testosterone deficiency.6
References
- Krausz C, Cioppi F. J Clin Med. 2021;10(17):4009. DOI.
- Brannigan RE, et al. J Urol. 2024;212(6):789-799. DOI.
- Eliveld J, et al. Hum Reprod Update. 2018;24(4):442-454. DOI.
- Miller N, et al. Fertil Steril. 2017;107(6):1305-1311. DOI.
- Durbin CG, et al. J Urol. 2025 May 1; 213(5S):e824. DOI.
- Hussein A, et al. BJU Int. 2013;111(3 Pt B):E110-E114. DOI.