Posterior urethral reconstruction in the context of radiation injury, fistula formation, and bony involvement represents one of the most challenging scenarios in urologic reconstruction. Traditional reconstructive techniques are often inadequate when the urethral defect is extensive and the tissue bed is fibrotic and avascular due to radiation.
This case of the month highlights an innovative bladder-sparing approach. A 55-year-old male presented with a 7-cm posterior urethral defect complicated by uro-symphyseal fistula and chronic osteomyelitis following pelvic radiation. Using single-port robotic surgery combined with microsurgical free tissue transfer, we successfully reconstructed the posterior urethra with a vascularized ileal Monti tube, avoiding the need for cystectomy and preserving bladder function.
We present this urethroplasty technique as a bladder-sparing alternative to cystectomy for select patients with complex, radiation-induced urethral injuries.
Case Highlights:
- The patient had been counseled for cystectomy with ileal conduit urinary diversion but wished to preserve his bladder.
- The procedure utilized a 20-cm segment of ileum, with the mesenteric vessels preserved for microvascular anastomosis.
- The ileal segment was divided to create a Monti tube for posterior urethral replacement and an intact conduit for ileal vesicostomy.
- Complete healing was achieved by three weeks, with successful stoma closure at eight weeks and resolution of chronic osteomyelitis.
Patient Case
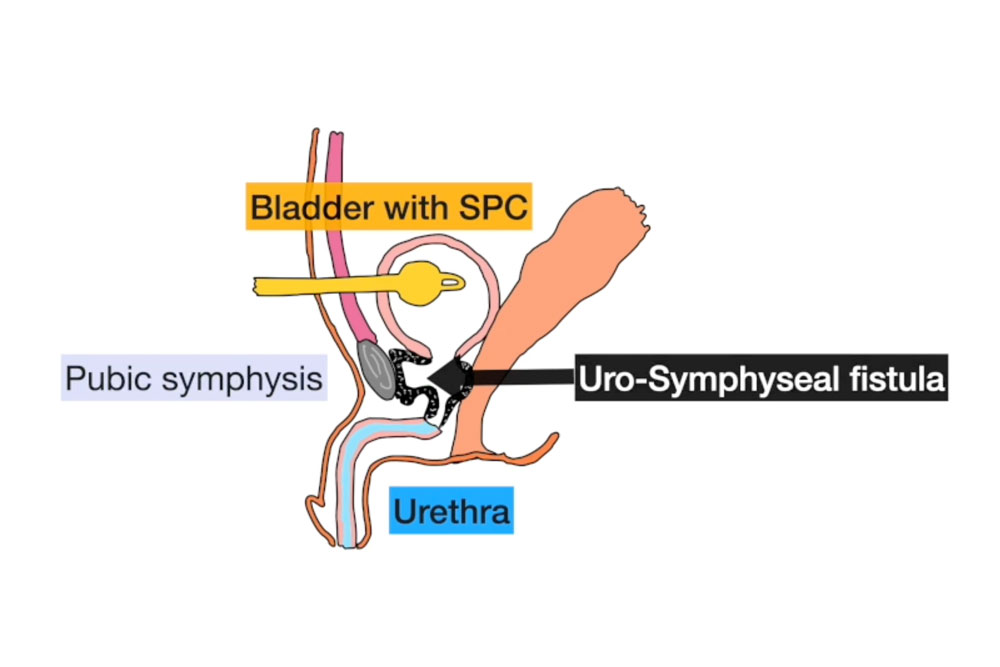
A 55-year-old male with a history of pelvic radiation therapy for prostate cancer presented with chronic pelvic pain, recurrent urinary tract infections, and severe lower urinary tract symptoms. He had been managed at an outside institution for years with progressive lower urinary tract dysfunction and was eventually diagnosed with a complex vesicourethral anastomotic stricture (VUAS) complicated by a uro-symphyseal fistula (USF) and chronic pubic osteomyelitis.
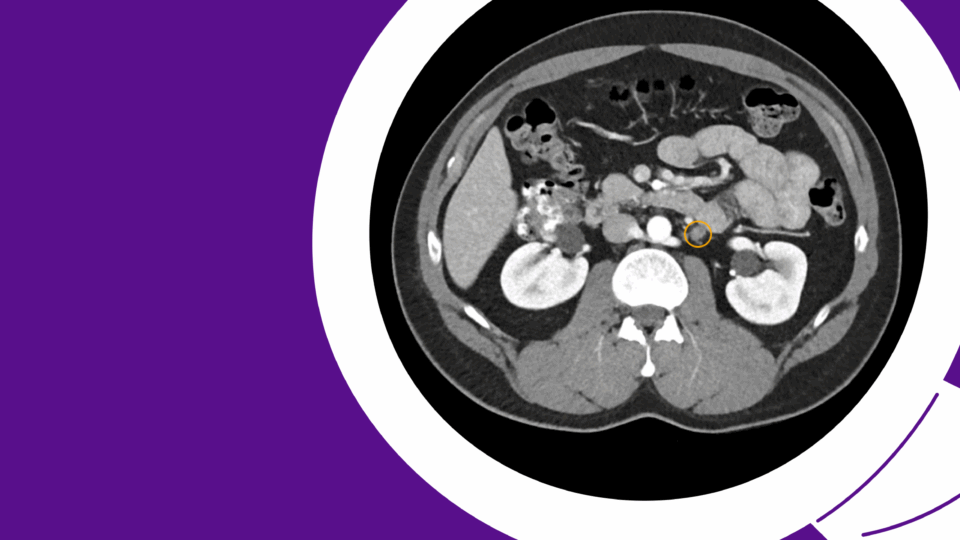
Imaging revealed extensive calcification and fibrosis involving the bladder neck, urethra, and pubic bone, with communication between the urinary tract and the pubic symphysis. Cystoscopy demonstrated a nearly obliterated posterior urethra, with an estimated 7 cm defect extending from the bladder neck to the bulbar urethra (Figure 1).
The patient had been previously counseled for cystectomy with ileal conduit urinary diversion, but he strongly desired to preserve his native bladder. He was referred to NYU Langone for evaluation of possible reconstructive options.
Management
Given the complexity of the defect, previous radiation, and active osteomyelitis, a multidisciplinary surgical plan was developed in collaboration with plastic surgery. The primary goal was to excise the necrotic tissue and fistula, reconstruct the urethral continuity using a well-vascularized substitute, and provide temporary urinary diversion to facilitate healing.
Surgical Approach: The procedure was performed under general anesthesia with the patient in Trendelenburg lithotomy position to facilitate simultaneous abdominal and perineal access.
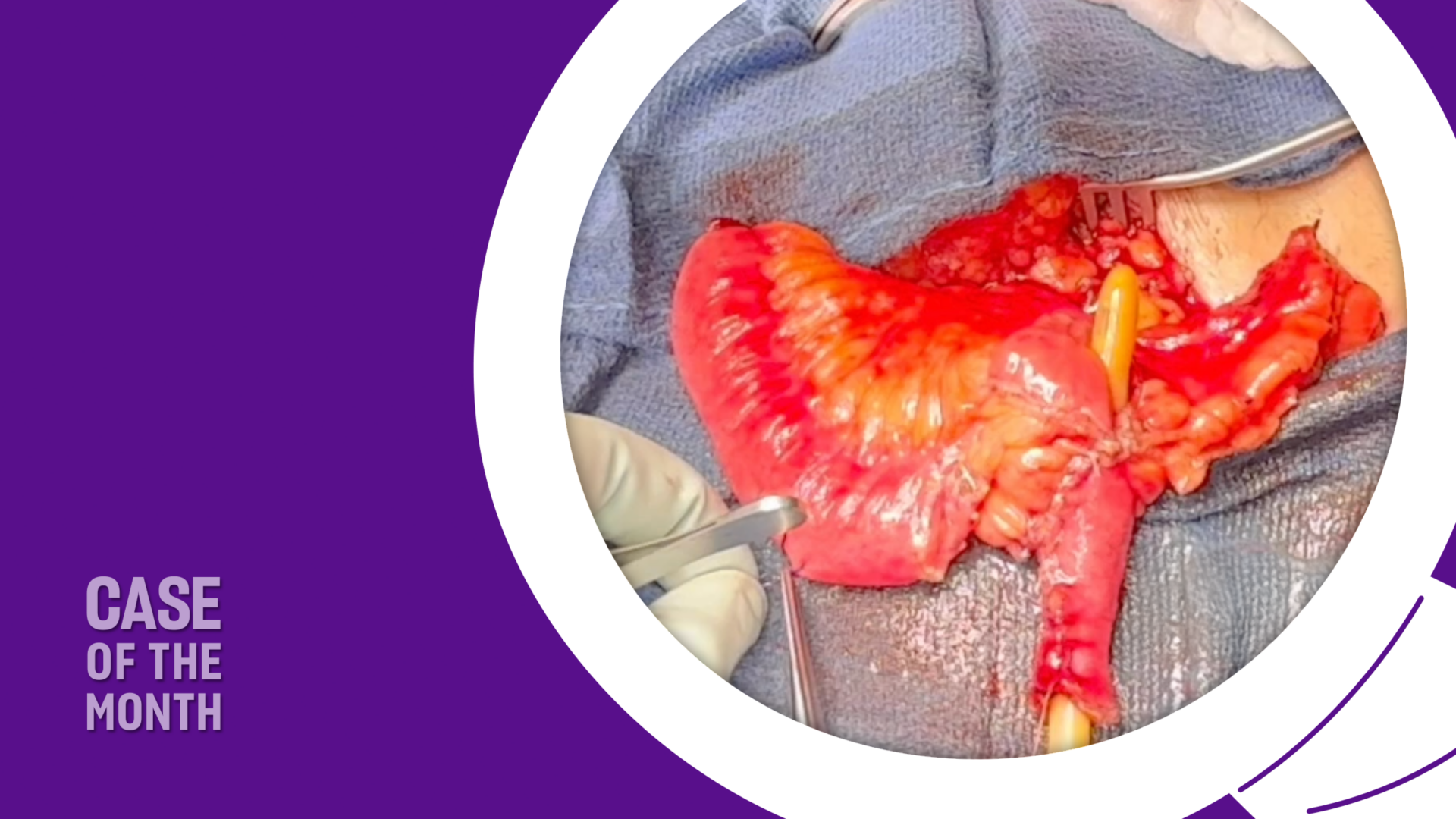
Robotic Access and Bladder Neck Dissection: A 3 cm incision was made in the right upper abdomen to dock the da Vinci SP® robotic system. The peritoneal cavity was entered, and extensive adhesiolysis was performed. The necrotic bladder neck tissue and fistulous connection to the pubic symphysis were sharply excised. A portion of the anterior bladder wall was also debrided.
Perineal Dissection: A midline perineal incision was made to expose the bulbar urethra, which was dissected free from the surrounding scar tissue. Proximal mobilization was achieved to enable a tension-free anastomosis with the planned flap.
Recipient Vessel Harvest: Using the single-port robot, the deep inferior epigastric vessels (DIEV) were dissected and delivered through a small lower midline incision (Figure 2). These vessels were selected to provide robust blood supply for the interposition flap.
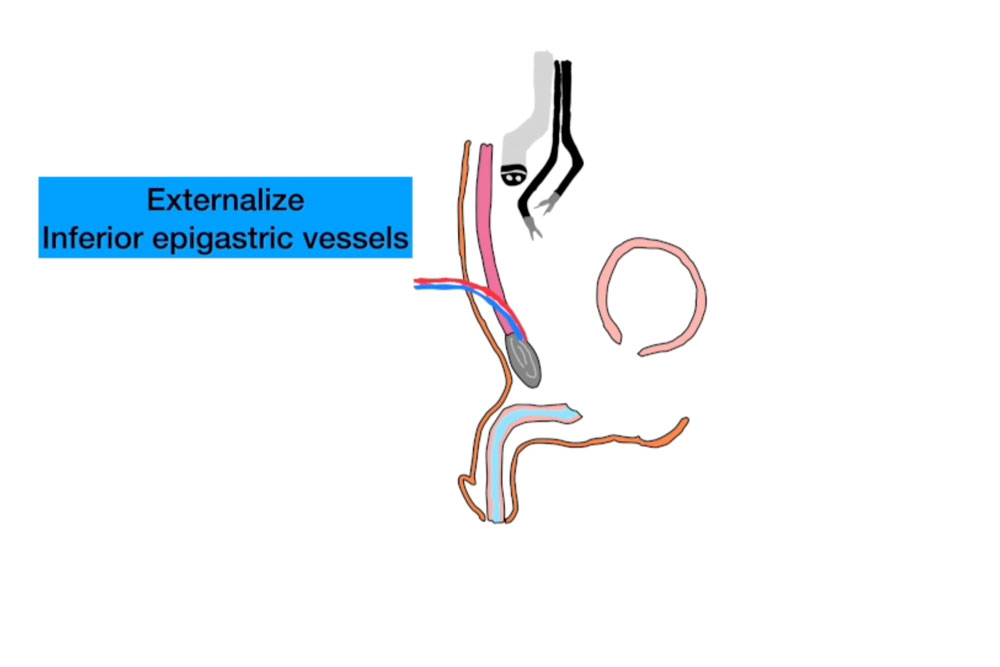
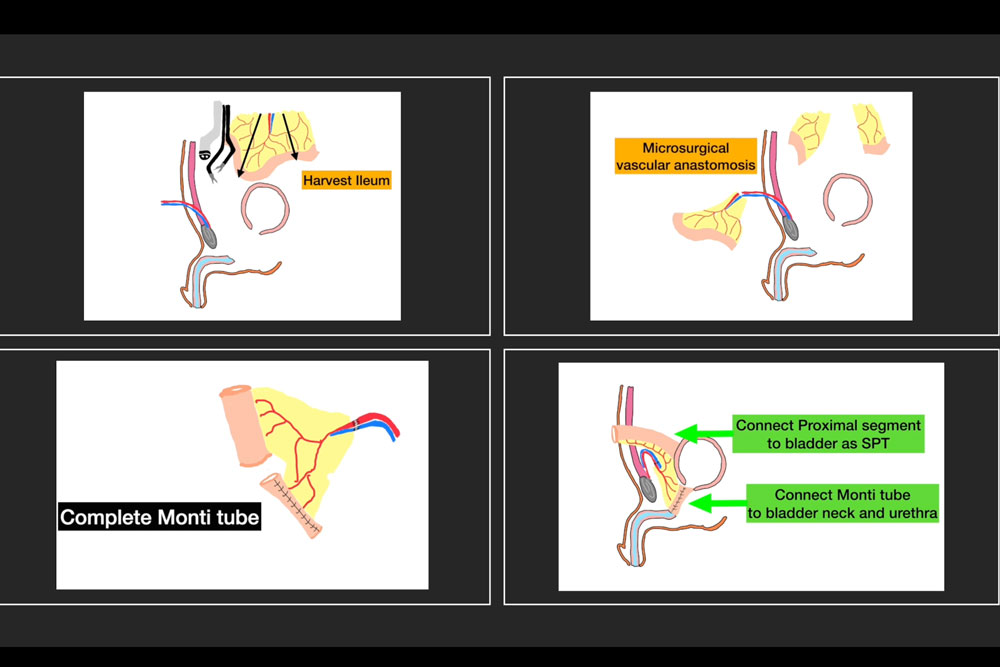
Free Flap Harvest and Microvascular Anastomosis: A 20 cm segment of ileum, located approximately 15 cm proximal to the ileocecal valve, was isolated and harvested. The mesenteric vessels were preserved for microvascular anastomosis. The bowel continuity was restored primarily. The harvested ileal segment was delivered through the lower midline incision.
Microsurgical anastomosis of the mesenteric artery and vein to the DIEV was performed by the microsurgery team under operative microscope. Excellent pulsatility and venous outflow were confirmed (Figure 3).

Flap Preparation and Reconstruction: The ileal segment was divided into two portions. The first 8 cm was tubularized into a spiral Monti tube to form a neourethra. The remaining portion was used to create a suprapubic ileal vesicostomy to decompress the bladder during the healing period and monitor the flap (Figure 4).
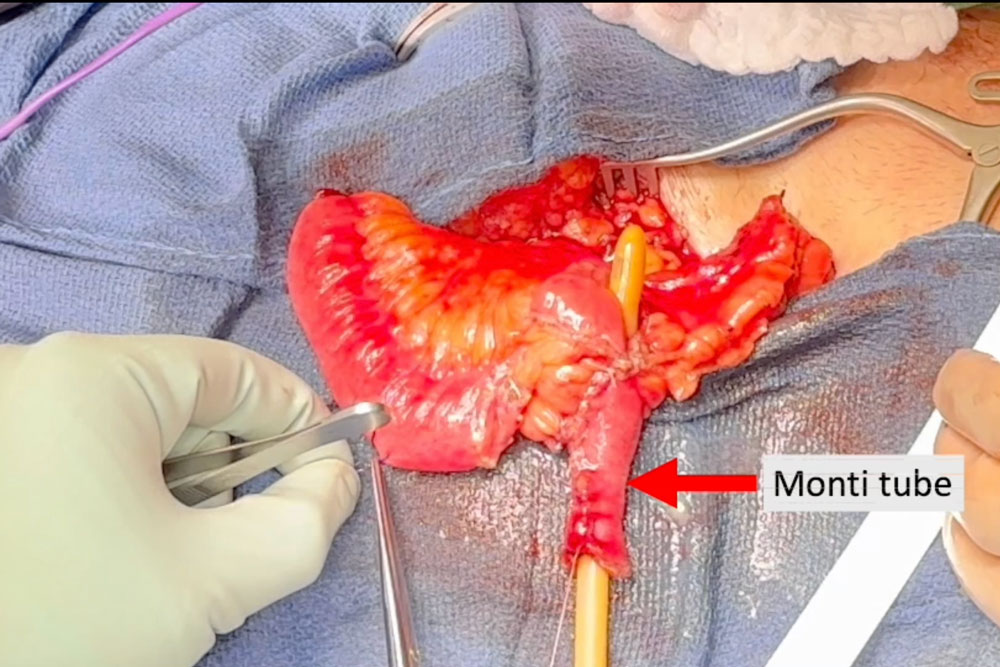
The Monti tube was delivered to the pelvis and the distal end was passed through the perineum. It was anastomosed proximally to the bladder neck and distally to the bulbar urethra in a tension-free, watertight manner using absorbable sutures.
Urinary Diversion: The remaining ileal segment was anastomosed to the bladder dome and the other end was brought up to the anterior abdominal wall and matured as a chimney vesicostomy to drain the bladder during the early postoperative period (Figure 5). This stoma allowed direct visualization of the flap’s mucosa to monitor vascular status using doppler in the early healing phase.
Postoperative Course and Follow-Up
The patient had an uneventful postoperative course. He was ambulating by postoperative day two and tolerating a regular diet by day three. He was discharged home on postoperative day five with a Foley catheter and the suprapubic ileal stoma draining adequately.
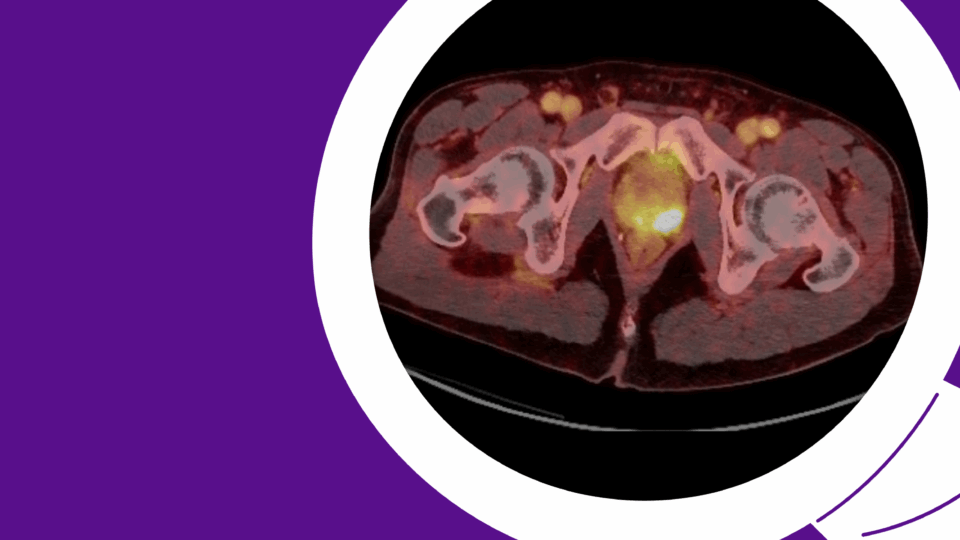
At three weeks, a voiding cystourethrogram (VCUG) confirmed complete healing of the anastomosis without extravasation. The urethral catheter was removed, and the patient resumed voiding per urethra with good flow. The suprapubic stoma was closed at eight weeks post-op after confirming stable bladder capacity and resolution of inflammation. Pain improved significantly, and follow-up imaging showed resolution of pubic osteomyelitis.
Conclusion
Single-port robotic-assisted posterior urethroplasty with tubularized ileal free flap interposition represents an advanced, bladder-sparing solution for managing devastating posterior urethral defects.
The use of free tissue transfer, particularly with intestinal segments, provides a vascularized, pliable conduit capable of spanning long defects. The spiral Monti flap, first described in pediatric urology, offers an ideal geometry and diameter for urethral substitution. The integration of robotic and microsurgical techniques, including use of the single-port robotic platform, allows safe access to the deep pelvis while minimizing morbidity in reoperative fields.
By combining the precision of robotic surgery with the versatility of microsurgical free tissue transfer, this technique provides a promising alternative to urinary diversion for select patients with complex pathology.
This case also highlights the importance of multidisciplinary collaboration, particularly with plastic and microsurgical expertise, to achieve functional reconstruction in patients who would otherwise be candidates for bladder removal and diversion.