Referral Notes:
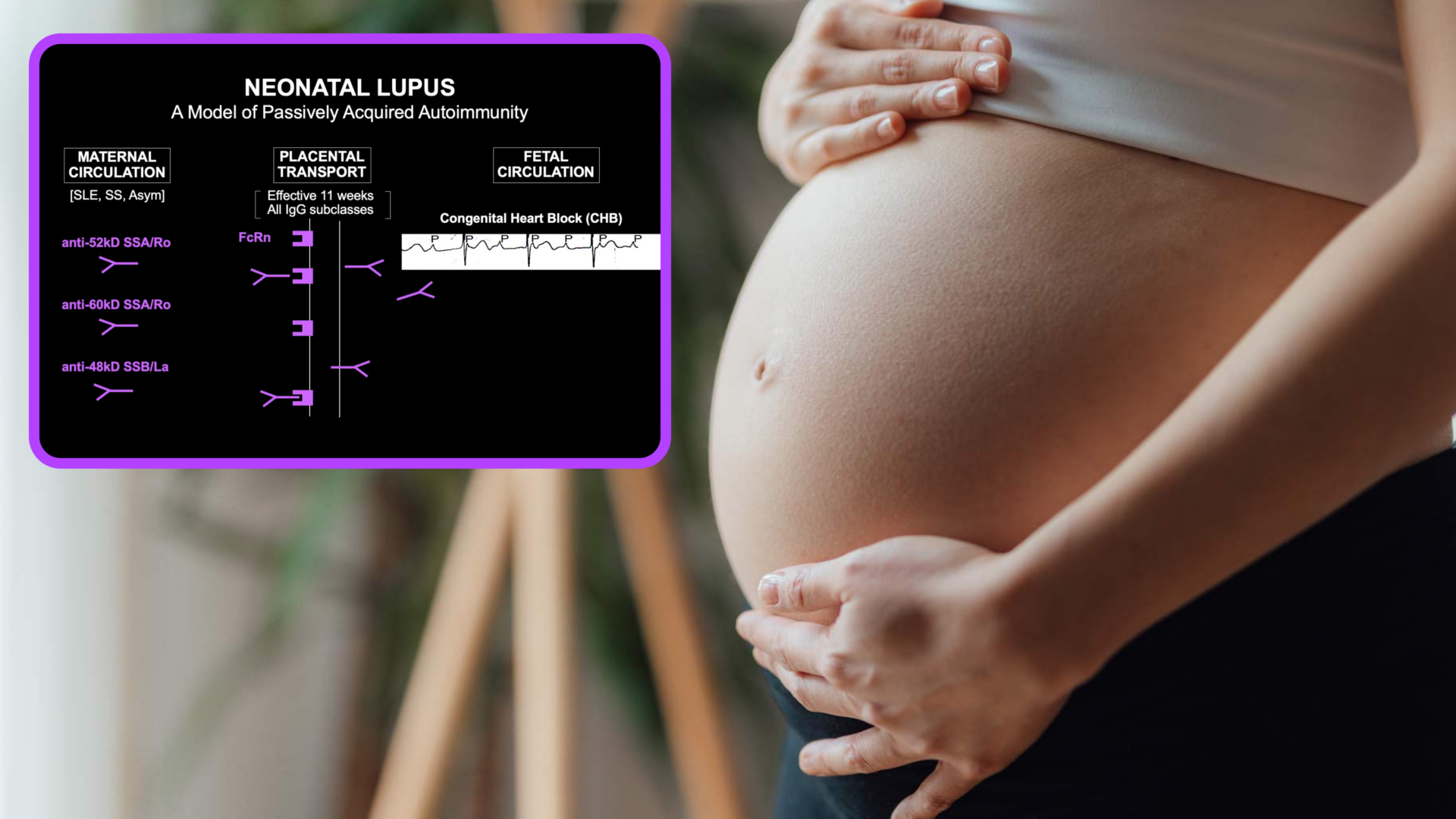
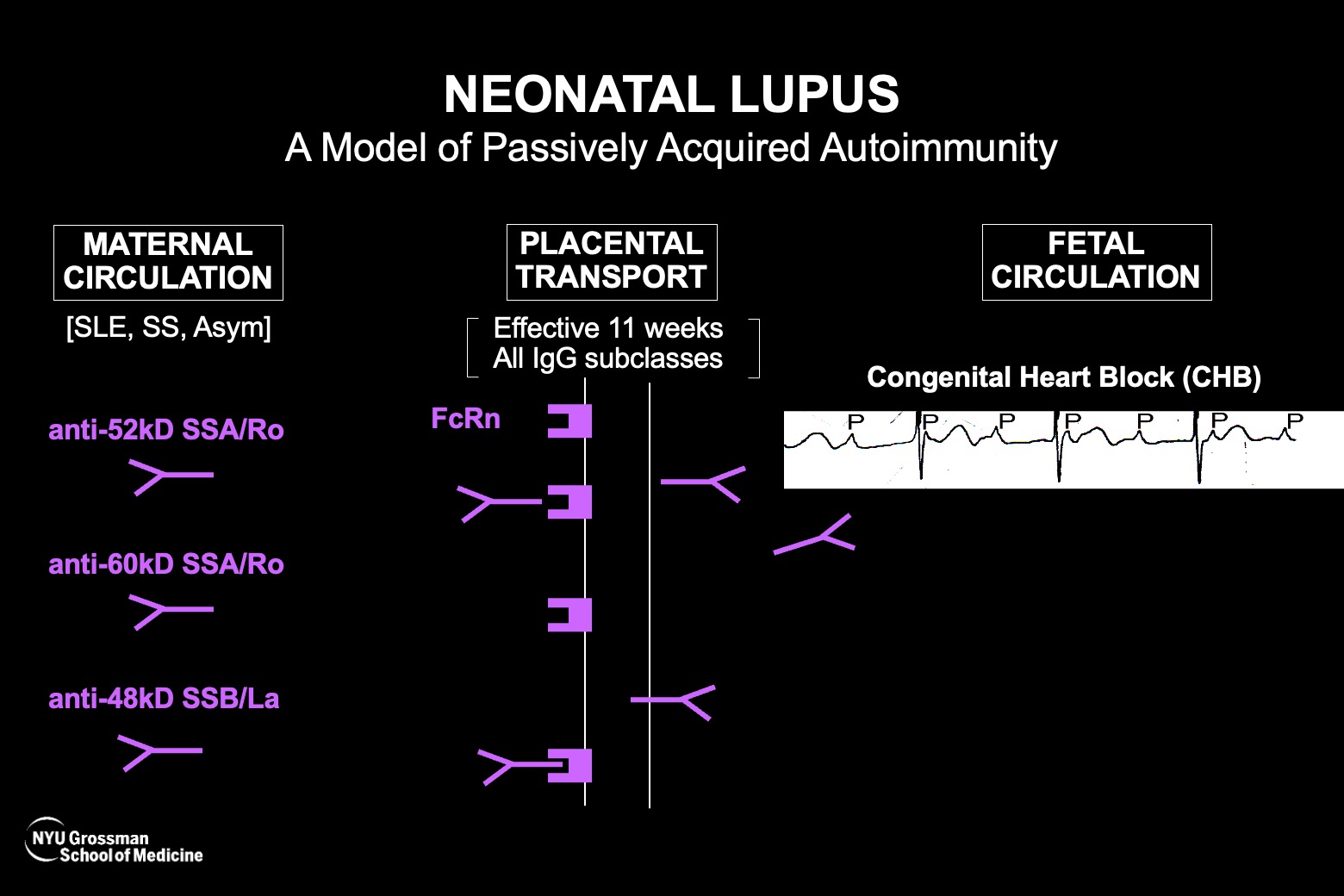
- Maternal anti-SSA/Ro antibodies are almost always present when cardiac neonatal lupus is diagnosed in a fetus during pregnancy. Thus, anti-SSA/Ro antibodies are necessary, but because the disease is so rare there must be amplifying factors; not all women with even the highest titer get disease.
- A new study confirms placental transfer of maternal anti-SSA/Ro antibodies into the fetal circulation in three fatal cases of cardiac neonatal lupus before the 25th week of gestation, establishing the previously unproven early transfer.
- The autoantibody levels found in the fetal circulation and/or pleural/pericardial fluid ranged between 6.5 and 15 percent of maternal levels, which still exceeded a known risk threshold for cardiac neonatal lupus.
- The findings support strategies to block placental transfer.
- Identifying amplifying factors is needed to help determine which patients with high anti-SSA/Ro antibodies require treatment to prevent transfer.
In cardiac neonatal lupus, accumulating research suggests that maternal anti-SSA/Ro antibodies can attack the developing fetal heart during the 18th to 25th weeks of gestation. The heart injury is most frequently manifest as complete atrioventricular block, which is fatal in about 20 percent of cases.
“When the block is third degree it is permanent and cannot be reversed ” says Jill P. Buyon, MD, the Sir Deryck and Lady Va Maughan Professor of Rheumatology and director of NYU Langone Health’s Lupus Center. “It can be fatal and even when the fetus survives, there is almost always the requirement of a lifelong pacemaker.”
Researchers have established that anti-SSA/Ro antibodies are almost always found when fetal heart block is identified in the second trimester. Even so, the presence of the autoantibodies alone, while seemingly necessary, is not sufficient since the fetal disease happens in 4 to 18 percent of those cases.
Perplexingly, although a very strong relationship exists between maternal anti-SSA/Ro autoantibodies and fetal heart block, the transport of both good and “bad” maternal antibodies across the placenta when the fetus is most vulnerable is hardly operative. Thus, proving that anti-SSA/Ro antibodies really do transfer so early in gestation has been a critical goal for researchers.
“For years, we’ve said that autoantibodies are highly associated and likely the cause of the disease, and that if there was no antibody, there would be no disease,” Dr. Buyon says. “But we hadn’t really proven that the antibody was in the fetal circulation that early on in pregnancy.”
Direct Direction of Autoantibodies
She and colleagues help resolve that question and open up novel prevention strategies in a new Lancet Rheumatology study published in collaboration with Justin S. Brandt, MD, director of the Division of Maternal–Fetal Medicine. From three fetuses in which the heart block proved fatal before the 25th week of development, the researchers were able to analyze umbilical cord and pericardial fluid, plus pleural fluid in one fetus.
“In all of these compartments, we identified that there was unequivocally maternal anti-SSA/Ro antibody present,” Dr. Buyon says. “This sets the story straight: not only did we detect the autoantibodies, but also at a level that theoretically could cause damage.”
“This sets the story straight: not only did we detect the autoantibodies, but also at a level that theoretically could cause damage.”
Jill Buyon, MD
The fetal autoantibody levels ranged between 6.5 and 15 percent of the maternal levels—in all three cases the mothers had very high levels and the fetal titers exceeded previously identified threshold levels for risk of cardiac neonatal lupus.
New Prevention Paradigm
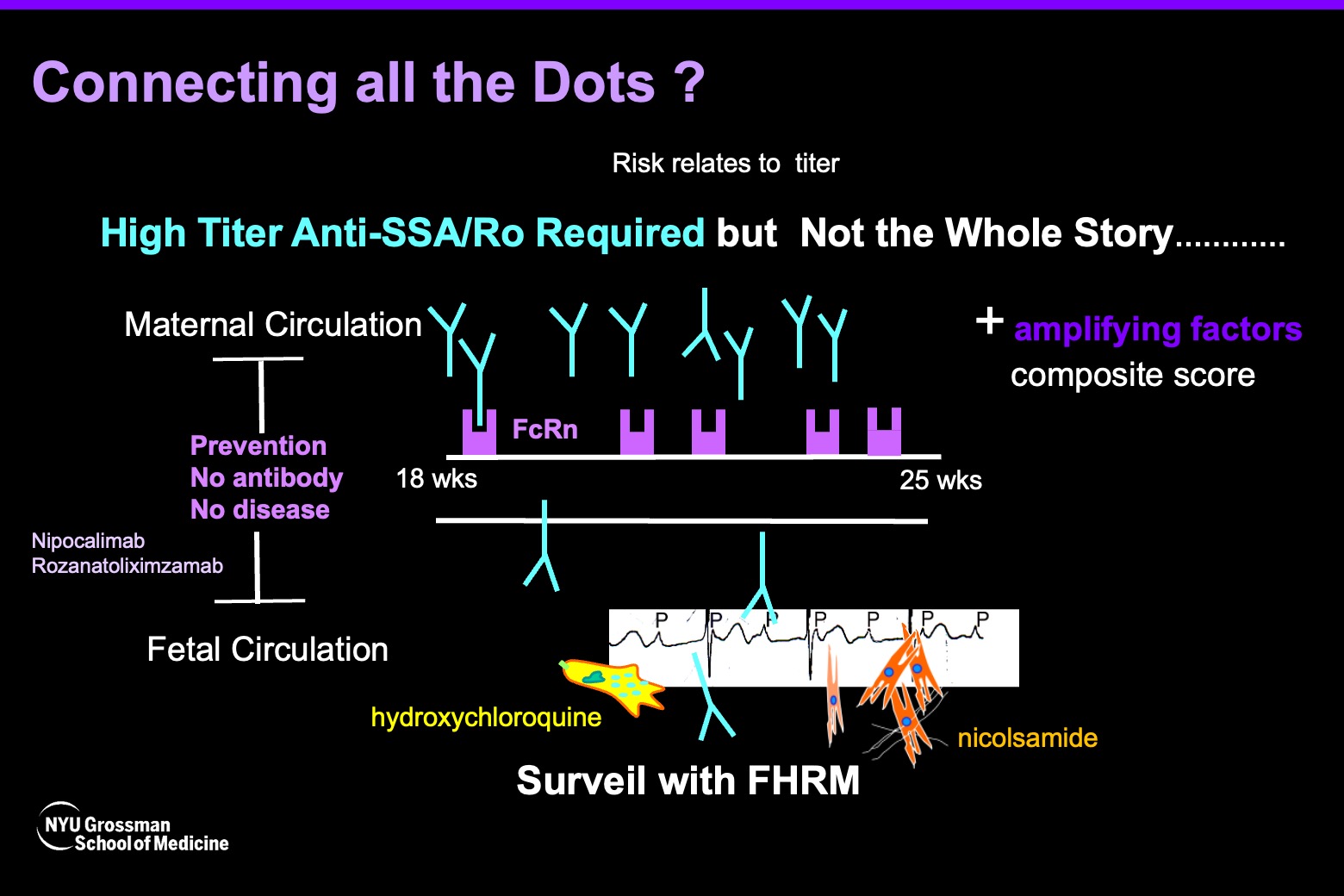
The study bolsters two new concepts regarding the disease, Dr. Buyon says. “First, it supports the importance of blocking placental transport of maternal antibodies as a prevention strategy.” In patients with high titers, reducing the maternal transfer from 15 percent to 1 percent or even zero could potentially eliminate the disease in the fetus, she says. This concept is now a reality with newly developed monoclonal antibodies that can block the placental receptors responsible for this transport.
The study, she says, also supports a second idea that low-titer maternal autoantibodies are probably not sufficient to cause the disease. “For someone who has very low autoantibody levels, if only 15 percent goes across to the fetus, maybe that wouldn’t be enough,” she says.
The findings, Dr. Buyon notes, were made possible by the three participating families and their desire to better understand the fatal disease, and by financial backing from donors Lauren and Andrew Levison in support of women’s health issues.
A Quest for Better Risk Detection
Determining who may be most at risk will depend upon further research. Because patients with systemic lupus erythematosus, Sjogren’s disease, or even rheumatoid arthritis can produce the anti-SSA/Ro antibodies, “it behooves rheumatologists to check for these autoantibodies as part of important pre-pregnancy counseling,” Dr. Buyon says.
Critically, however, even individuals without a diagnosed rheumatologic disease who have only minor symptoms, as in the case of the three participants in the new study—can produce high autoantibody titers. Even totally asymptomatic individuals can generate anti-SSA/Ro antibodies, says Dr. Buyon, with frequencies approaching 1 percent.
“The mother’s disease state does not influence whether the heart block happens; this is strictly an autoantibody-associated problem.”
“The mother’s disease state does not influence whether the heart block happens; this is strictly an autoantibody-associated problem,” she says. “This begs the question of whether anti-SSA/Ro antibody testing should become part of a pregnancy workup in otherwise healthy women.” She and Dr. Brandt are hoping to explore that possibility.
“I would argue, we now know that titer matters. About a third of people who have anti-SSA/Ro antibodies have low titer, so they likely wouldn’t have to worry,” Dr. Buyon says. For the remaining two-thirds of individuals, additional research could help identify factors that further amplify the risk of cardiac neonatal lupus conferred by high-titer anti-SSA/Ro antibodies. These findings have emerged from Dr. Buyon’s ongoing trial in collaboration with Bettina Cuneo, MD, of the University of Arizona College of Medicine, titled “Surveillance and Treatment tO Prevent Fetal Atrioventricular Block Likely to Occur Quickly (STOP BLOQ).”
“If you could block the antibody that’s necessary for the disease, then the amplifying factors may not matter. But because overall cardiac neonatal lupus is rare even in those with the highest titers of anti-SSA/Ro antibodies, we need to find those other factors to identify who needs the therapy to get rid of the necessary autoantibodies,” she says.
At NYU Langone, Dr. Buyon has established the largest extant registry of subjects with high-titer anti-SSA/Ro antibodies who have had affected and unaffected pregnancies, which should help to further advance her quest to eliminate this devastating lifelong disease.